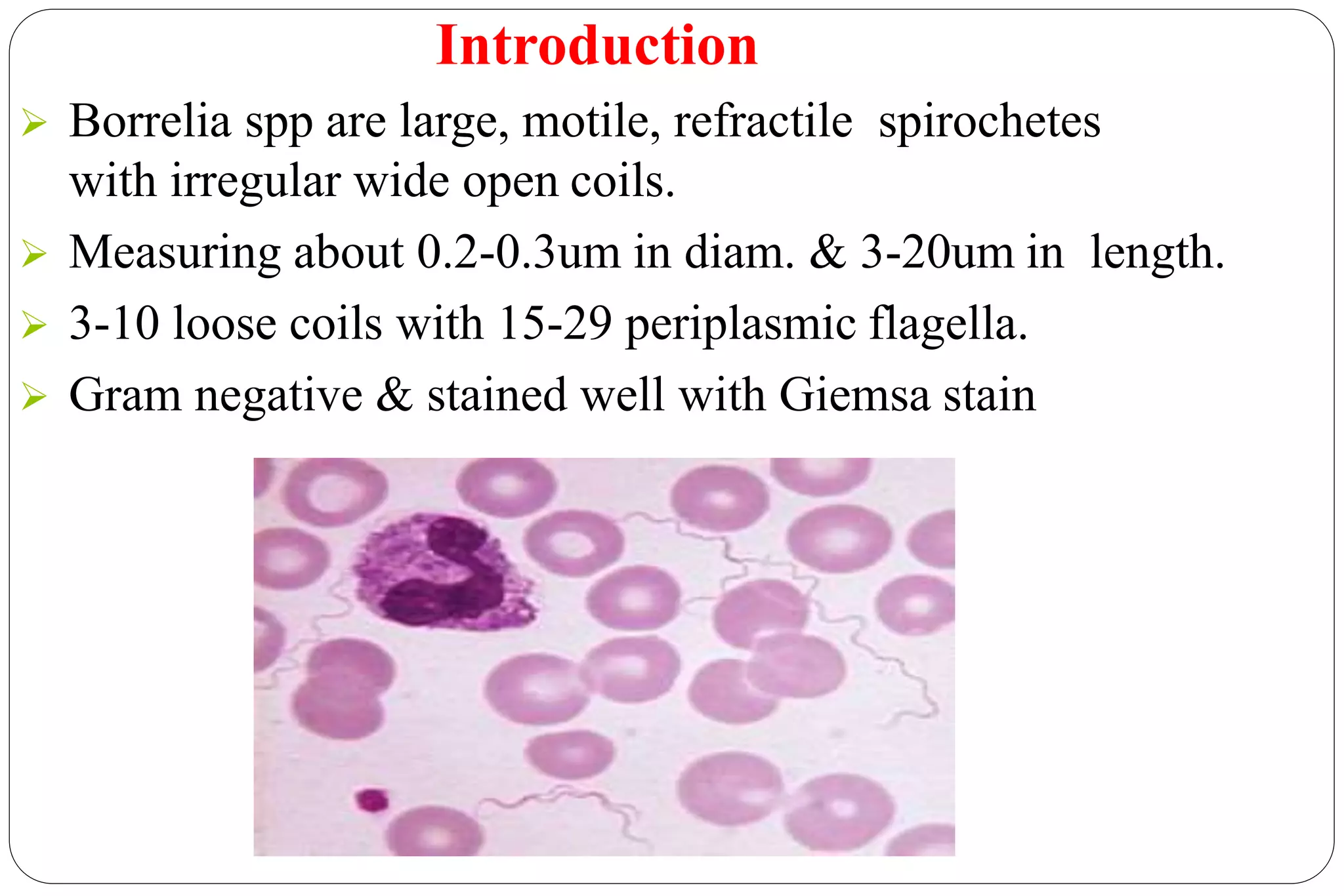
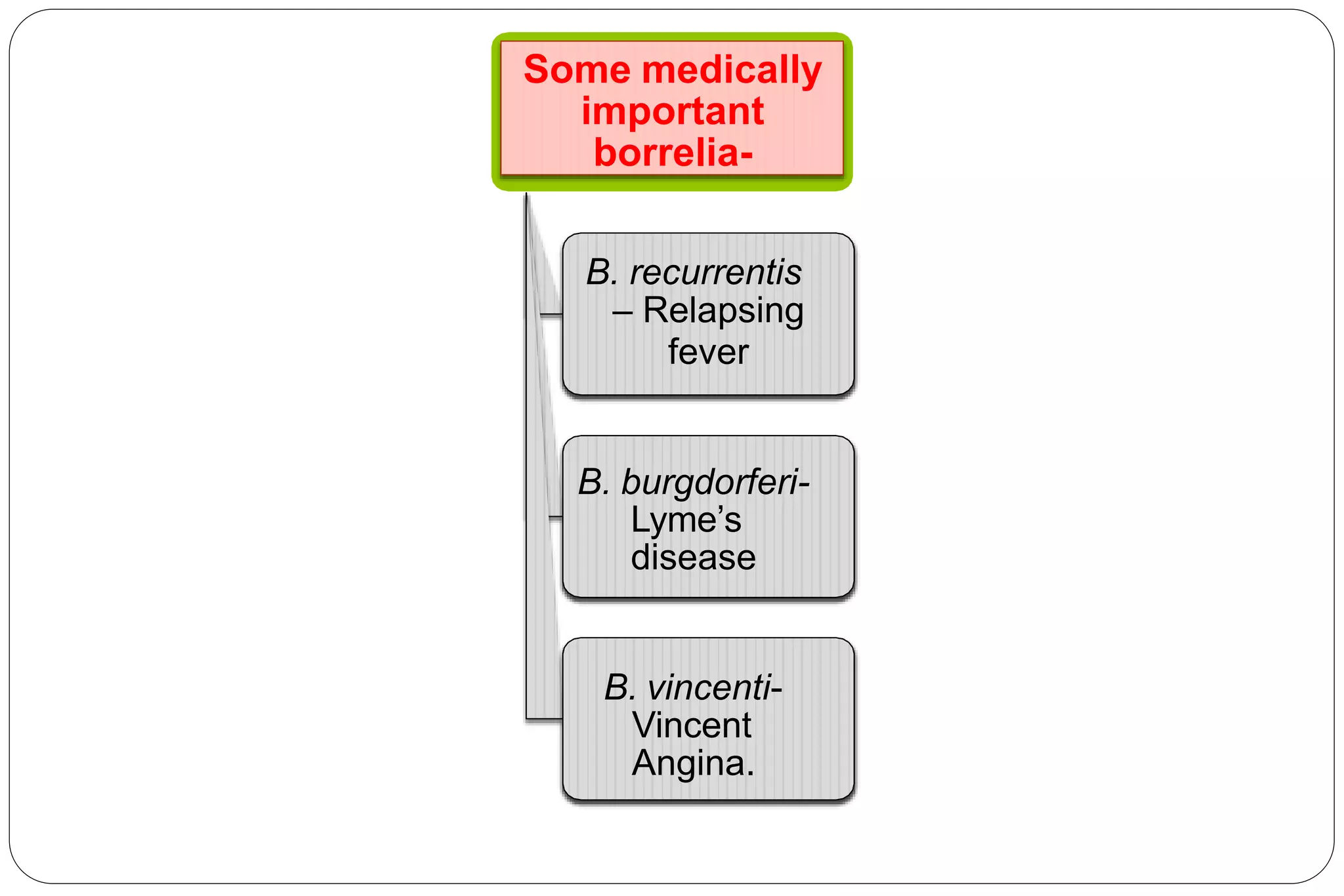
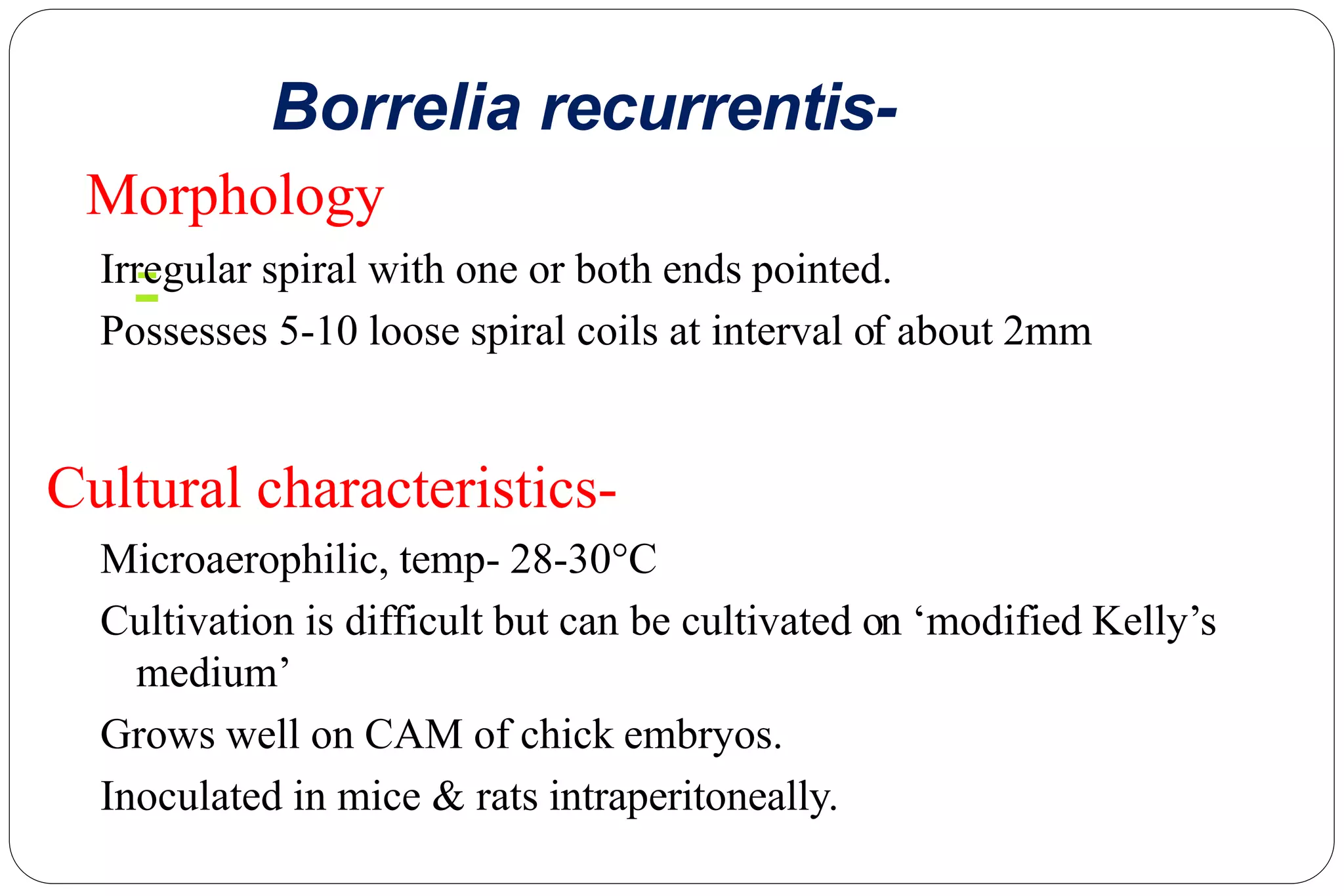
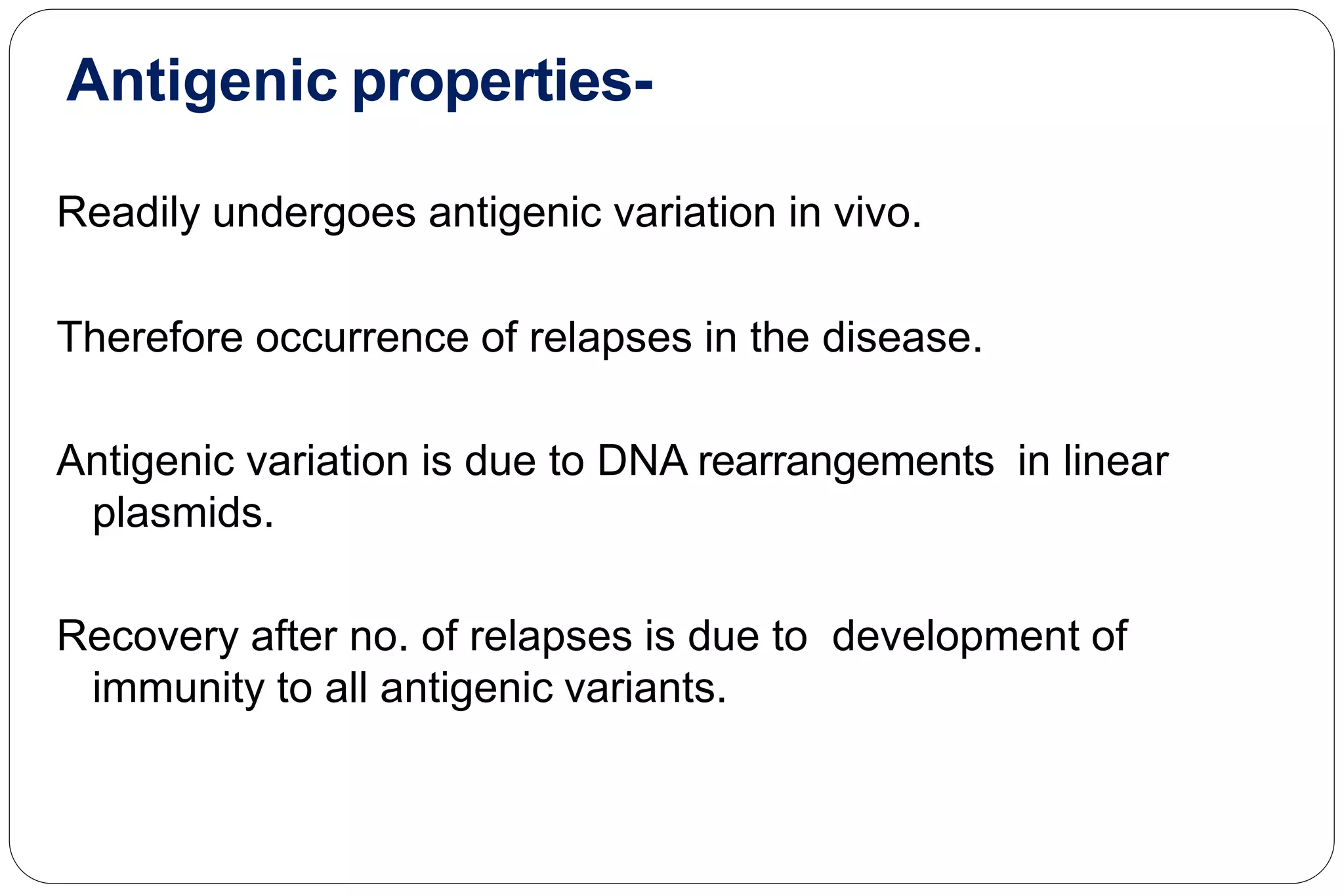
This document provides information about the bacteria Spirochaetes. It discusses their morphology, motility, reproduction, pathogenic species, diseases caused, and laboratory diagnosis. Some key points:
- Spirochaetes are elongated, helically coiled, and motile bacteria with endoflagella. They can cause diseases like syphilis, Lyme disease, and leptospirosis.
- Pathogenic species include Treponema pallidum (syphilis), Borrelia burgdorferi (Lyme disease), and Leptospira interrogans (leptospirosis).
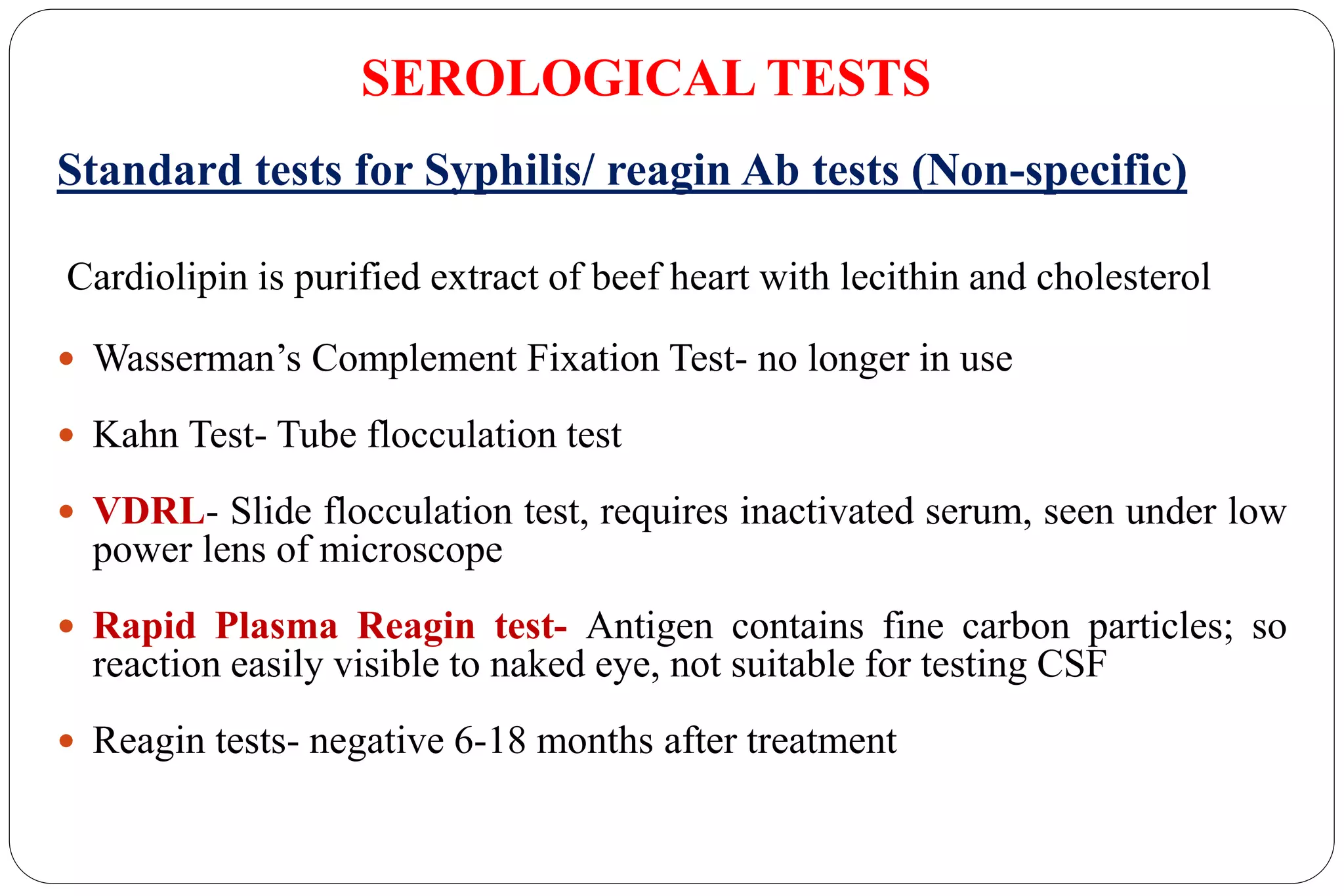
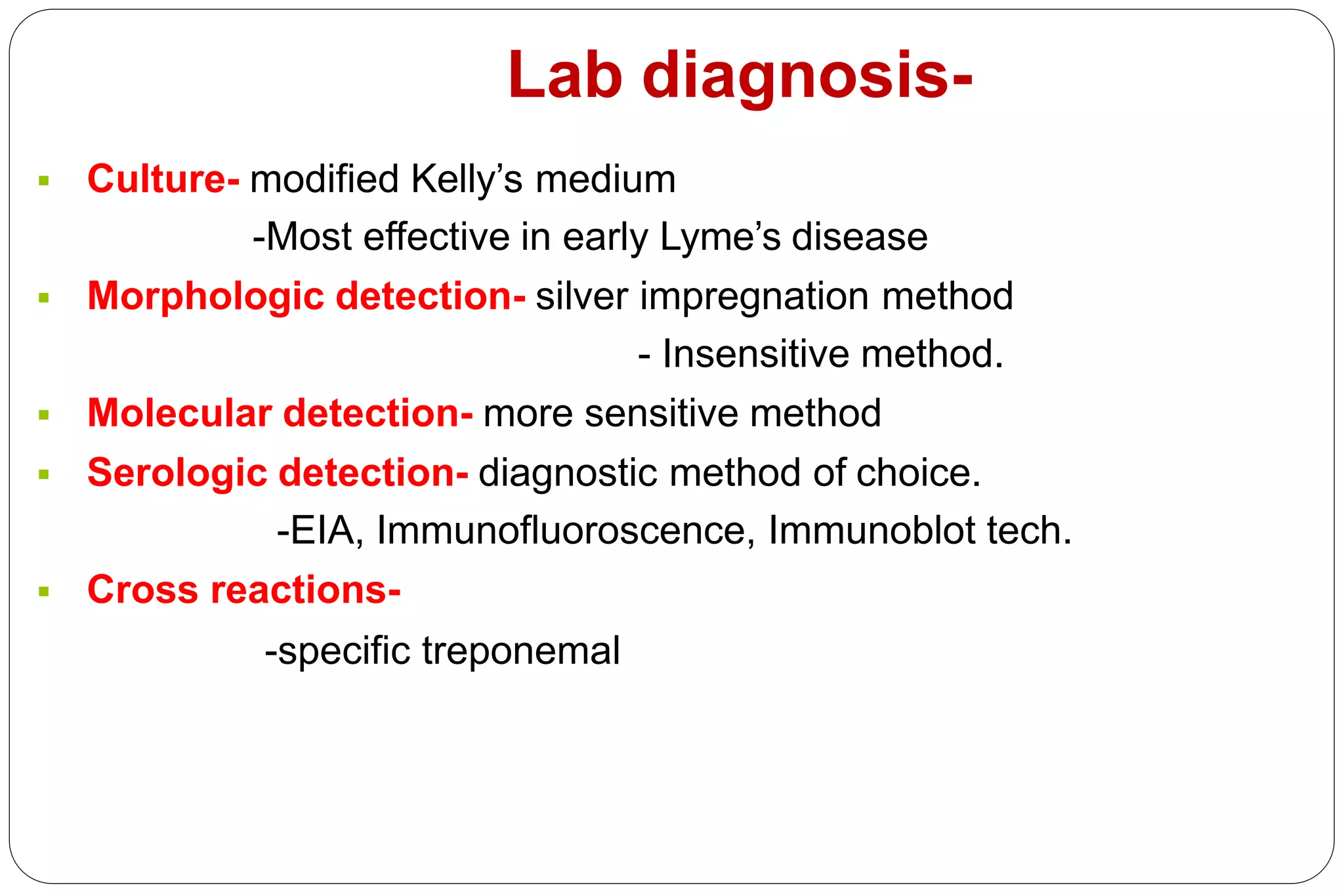
- Laboratory diagnosis involves darkfield microscopy, culture, serology, and