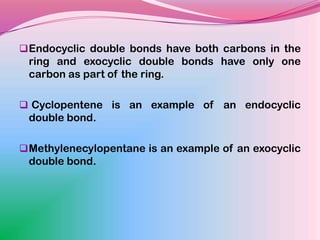
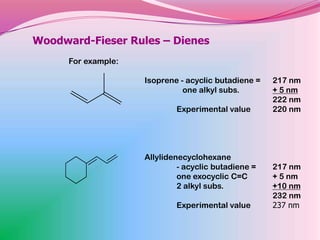
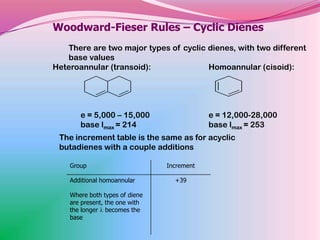
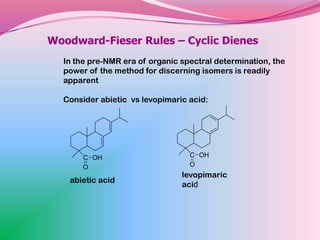
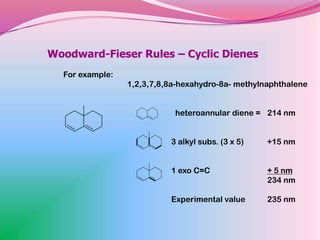
The document summarizes a seminar on applying the Woodward-Fieser rules to predict absorption maxima (λMax) of organic compounds. The rules were developed based on extensive studies of terpene and steroidal alkenes. They allow predicting λMax to within 5-6% based on structural features like conjugation, cyclicity, and substituents. The rules are useful for distinguishing isomers in the absence of NMR. Examples are given to demonstrate applying the rules for dienes, polyenes, and unsaturated carbonyl compounds.