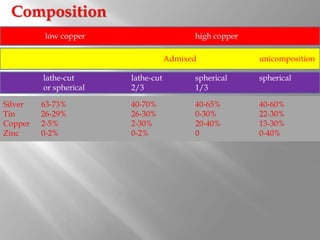
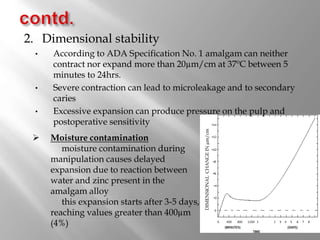
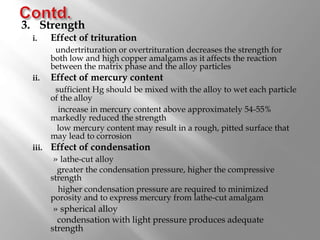
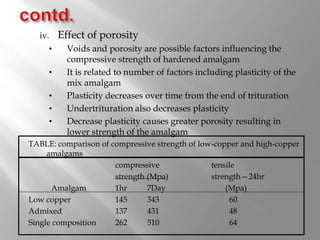
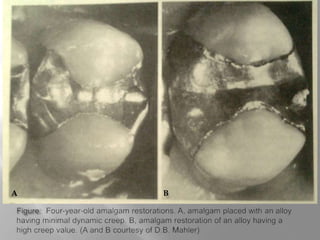
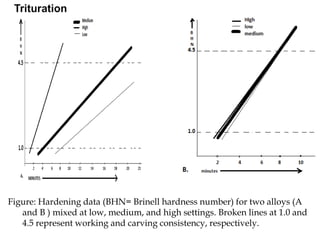
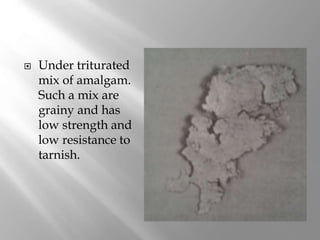
Dental amalgam is an alloy used in dentistry as a filling material. It contains mercury and other metals such as silver, tin, and copper. Amalgam is used for fillings in back teeth and to restore crowns. There are different types of amalgam based on their composition and particle shape. The properties and performance of amalgam depend on factors like mercury content, alloy composition, trituration, and condensation technique. While amalgam is inexpensive and durable, it also has disadvantages like poor aesthetics, potential toxicity, and marginal breakdown over time.