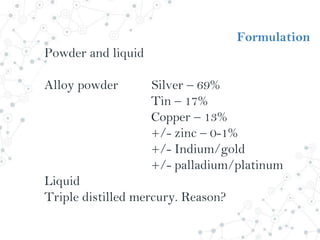
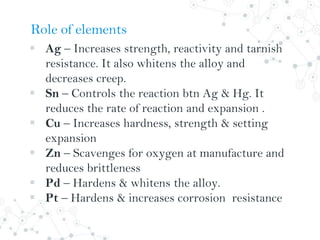
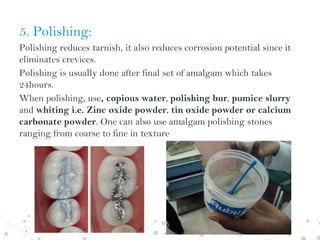
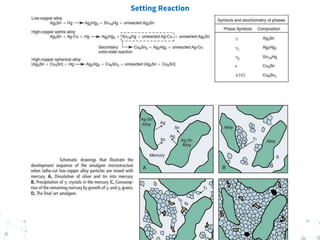
Dental amalgam is an alloy used in dental restorations composed of silver, tin, copper and sometimes zinc, mixed with liquid mercury. The document outlines the formulation, classification, setting reaction and properties of dental amalgam. It discusses factors that affect the strength and longevity of restorations, such as trituration technique and condensation pressure. While concerns exist regarding mercury toxicity, advocates argue that with proper handling techniques exposure is minimal, and no safer and more durable alternative has been found.