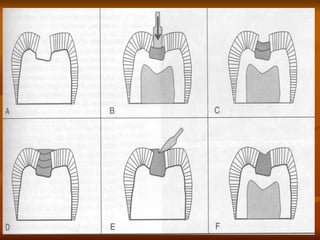
Dental amalgam is an alloy used as a dental restorative material. It consists of mercury combined with other metals like silver, tin, and copper. Amalgam undergoes a setting reaction when mixed with liquid mercury to form a hard material. It is indicated for restoring cavities. While it has advantages like strength and cost-effectiveness, it lacks esthetics and can release low levels of mercury vapor. Modern amalgams have improved properties like reduced creep and shrinkage. Careful manipulation is required to achieve optimal physical properties and reduce risks.