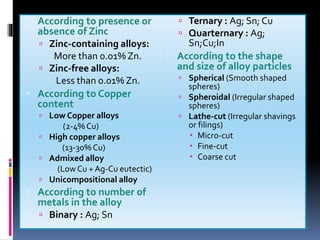
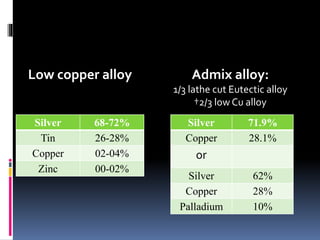
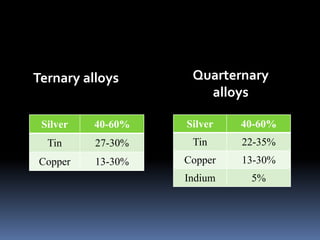
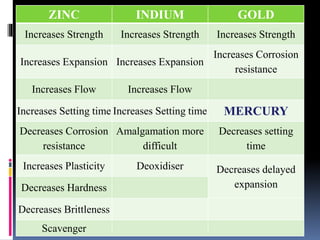
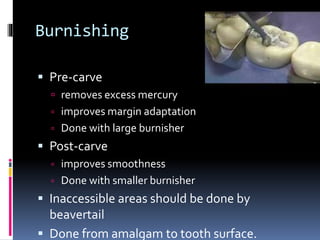
This document provides an overview of dental amalgam, including its:
- Classification based on composition and particle shape
- Generations and typical compositions
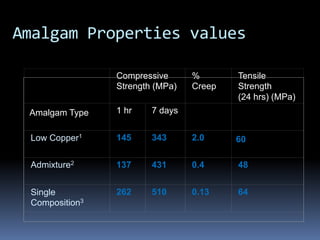
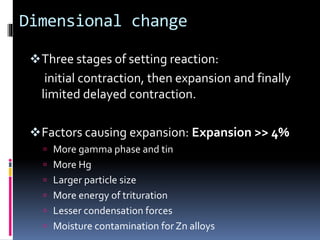
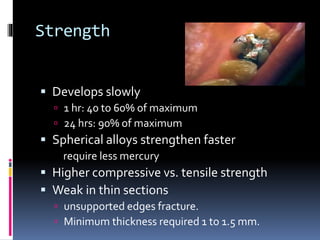
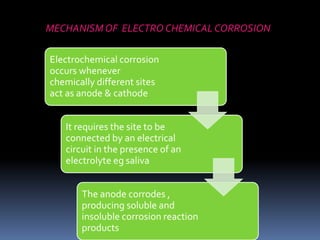
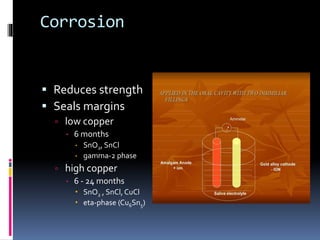
- Properties including strength, creep, corrosion resistance
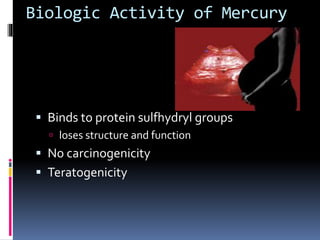
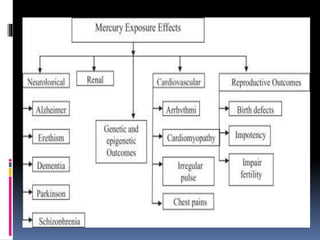
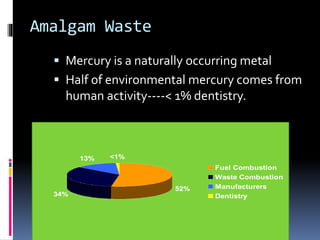
- Toxicity and mercury levels
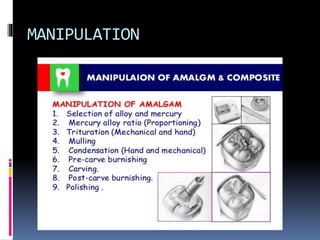
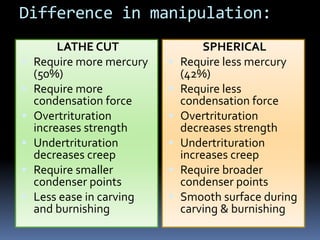
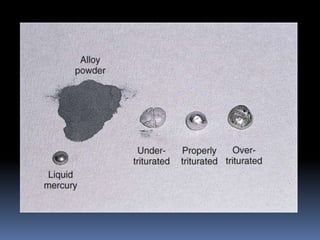
- Manipulation techniques for mixing, condensing, and finishing amalgam restorations
- Status and concerns about mercury levels from dental offices