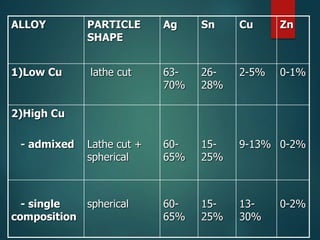
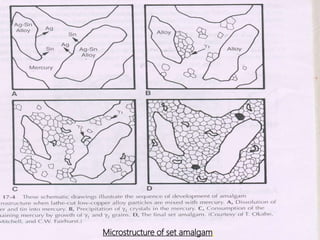
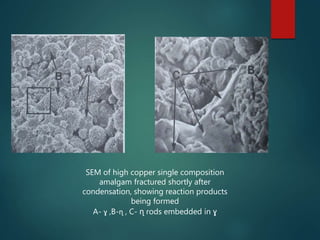
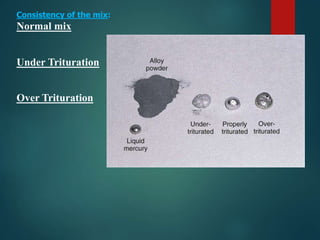
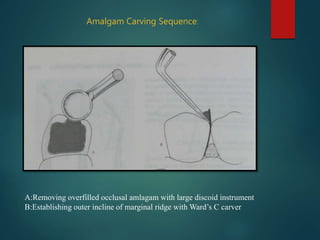
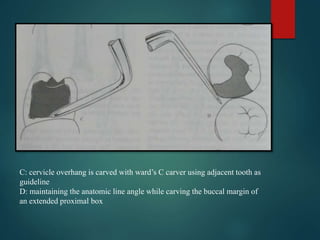
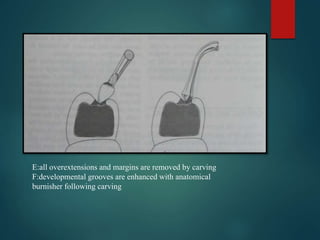
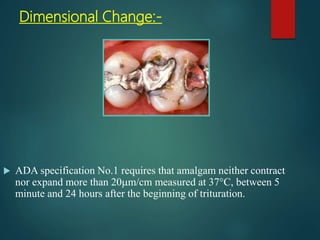
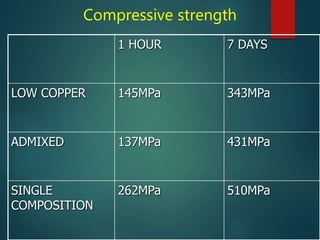
This document provides an overview of dental amalgam. It discusses the history, definitions, classifications, composition, reactions, technical considerations, and clinical uses of amalgam. Amalgam is an alloy of silver, tin, copper, and sometimes zinc that is mixed with liquid mercury to form a plastic mass that can be condensed into a cavity preparation. It sets via a chemical reaction between the alloy particles and mercury. Amalgam has good compressive strength and is used for moderate to large restorations, especially when esthetics is not a primary concern. Proper manipulation including trituration, condensation, and carving is required to achieve optimal physical properties and clinical performance.