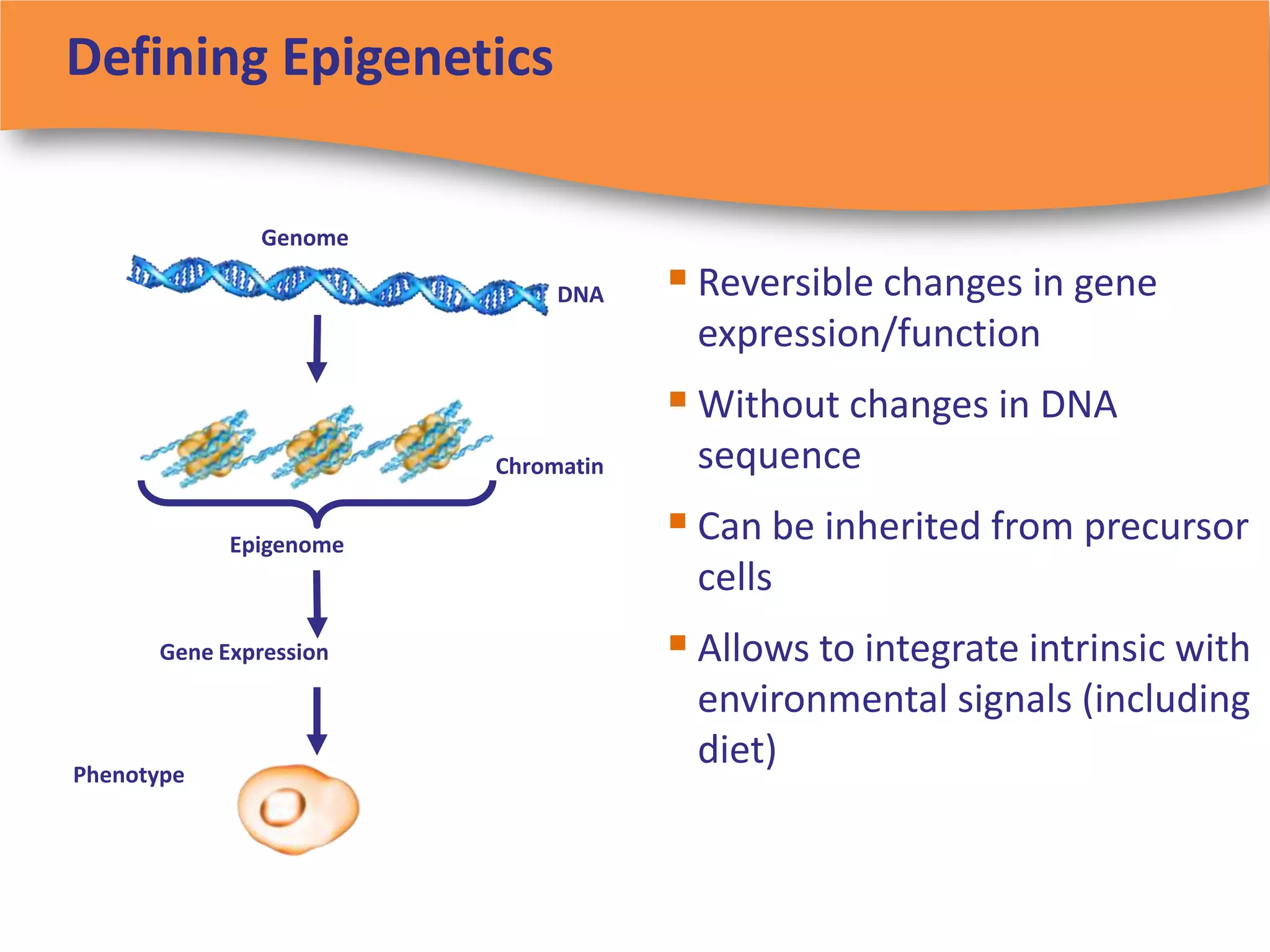
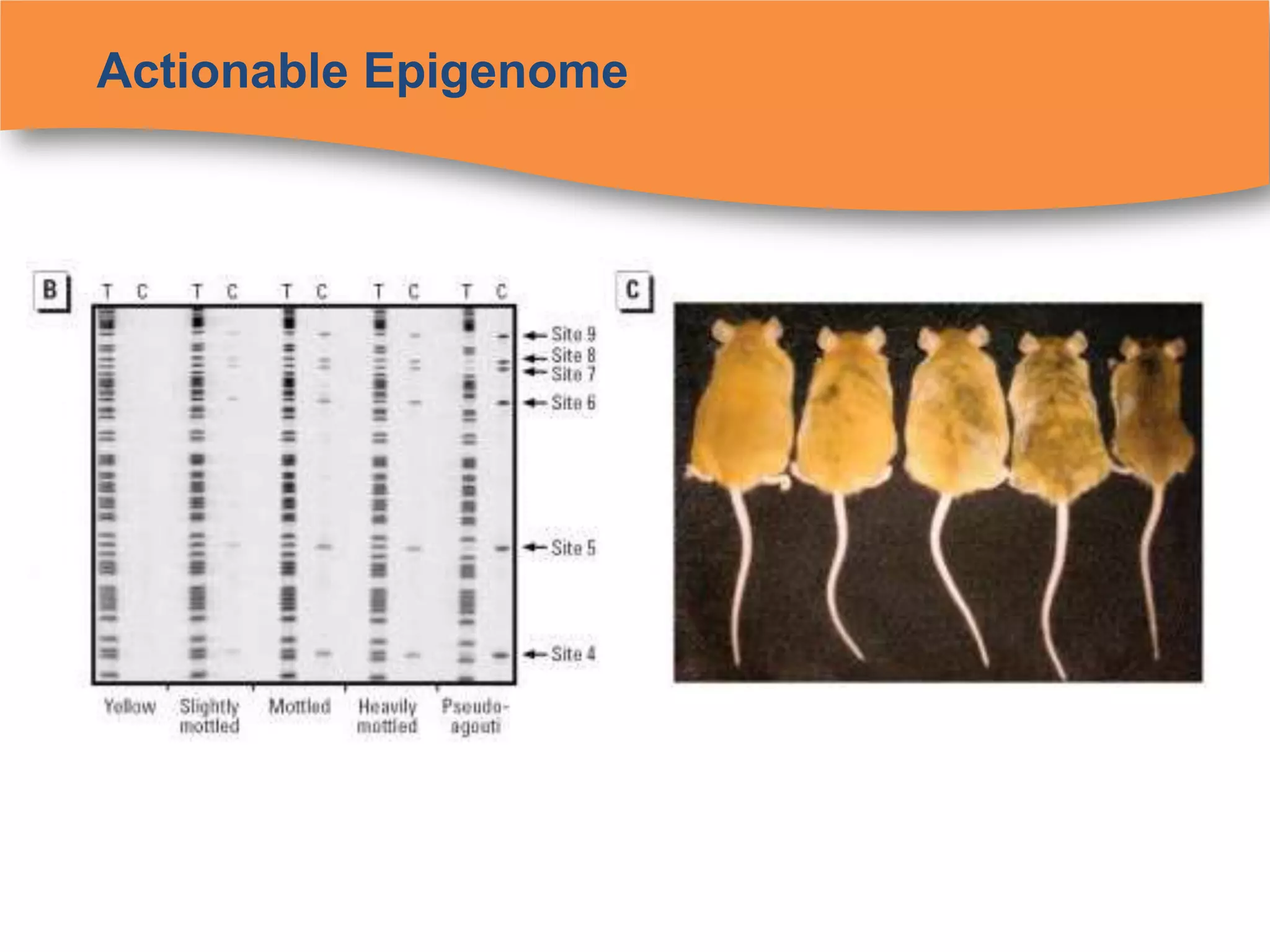
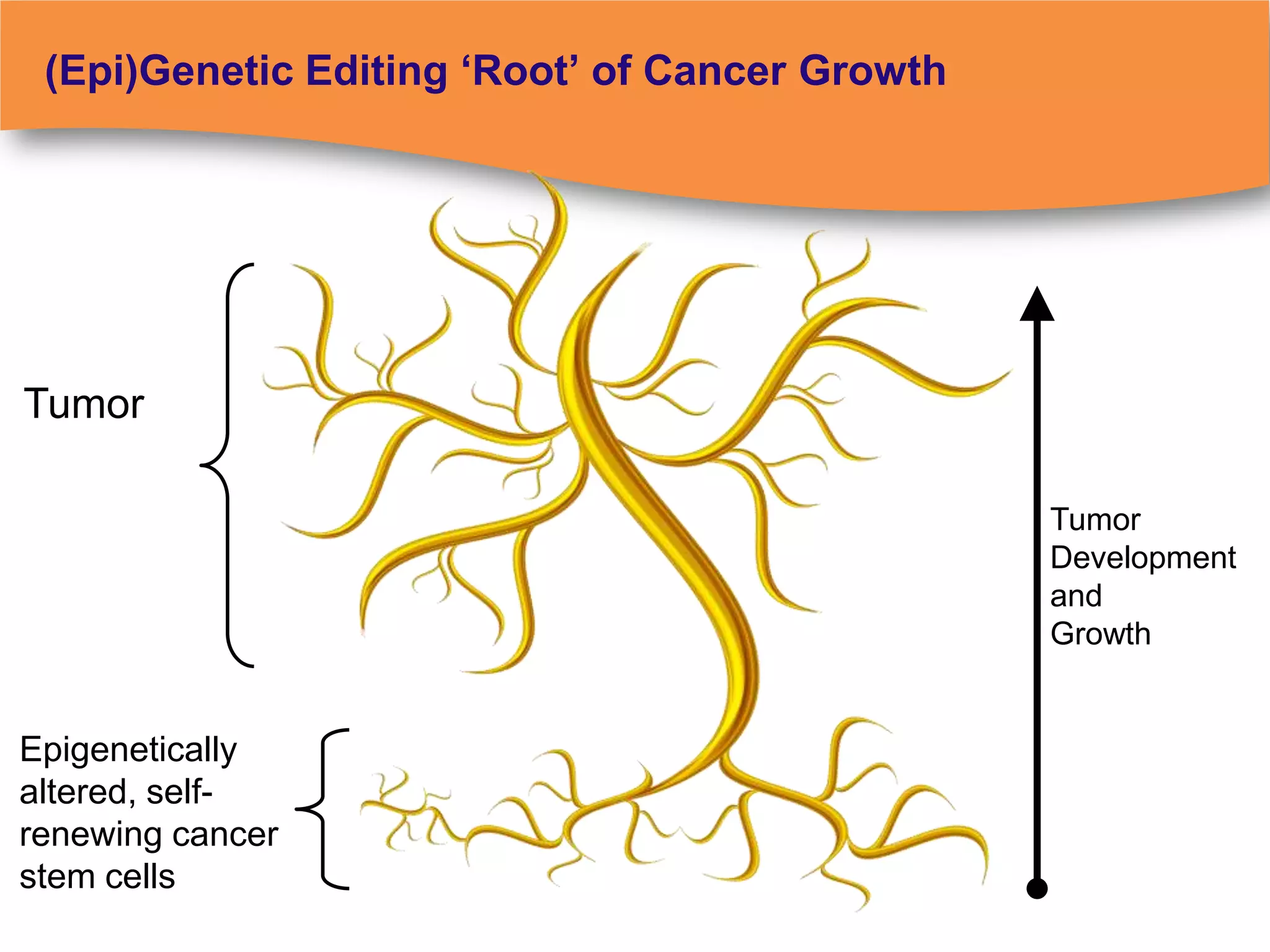
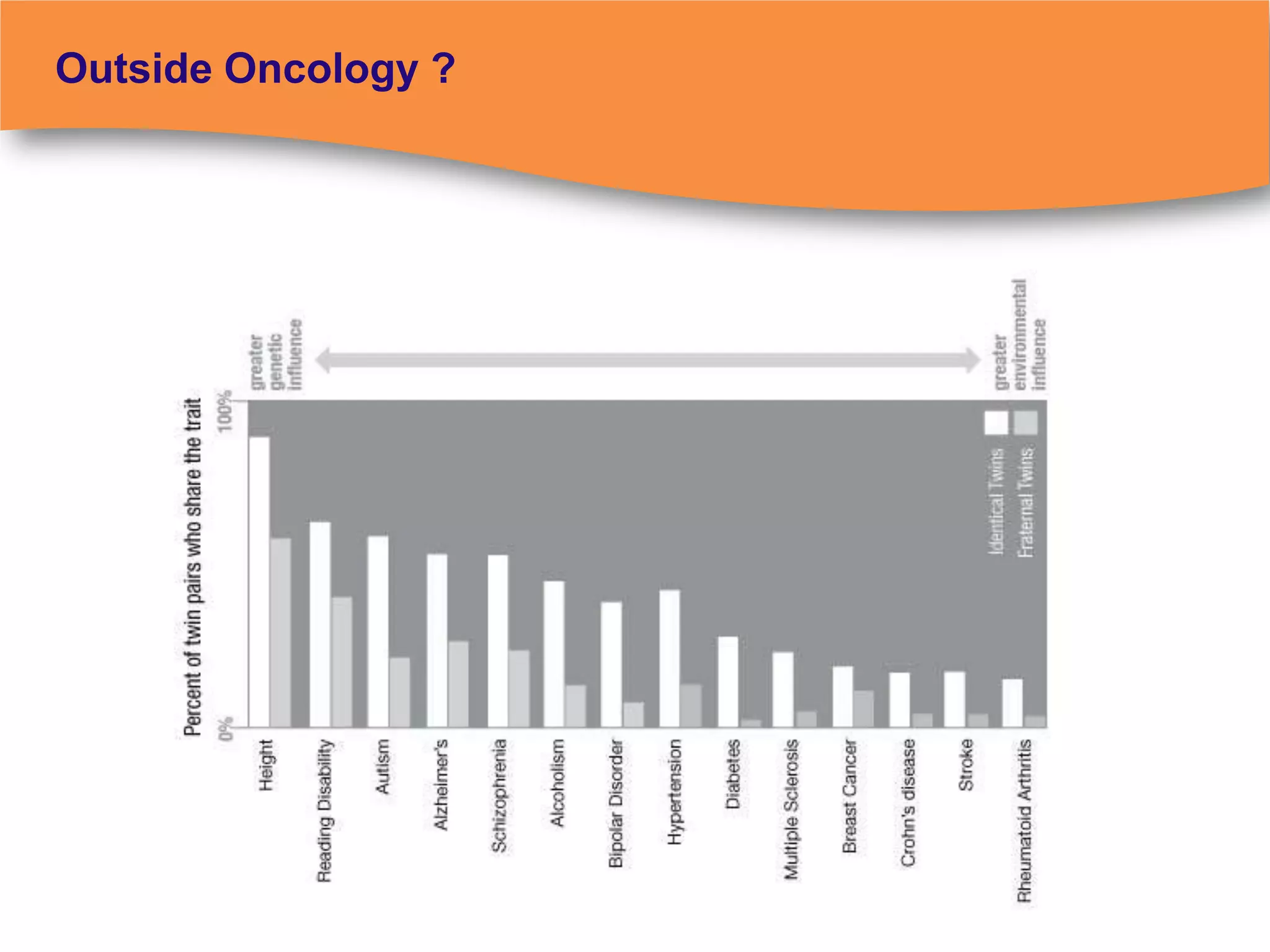
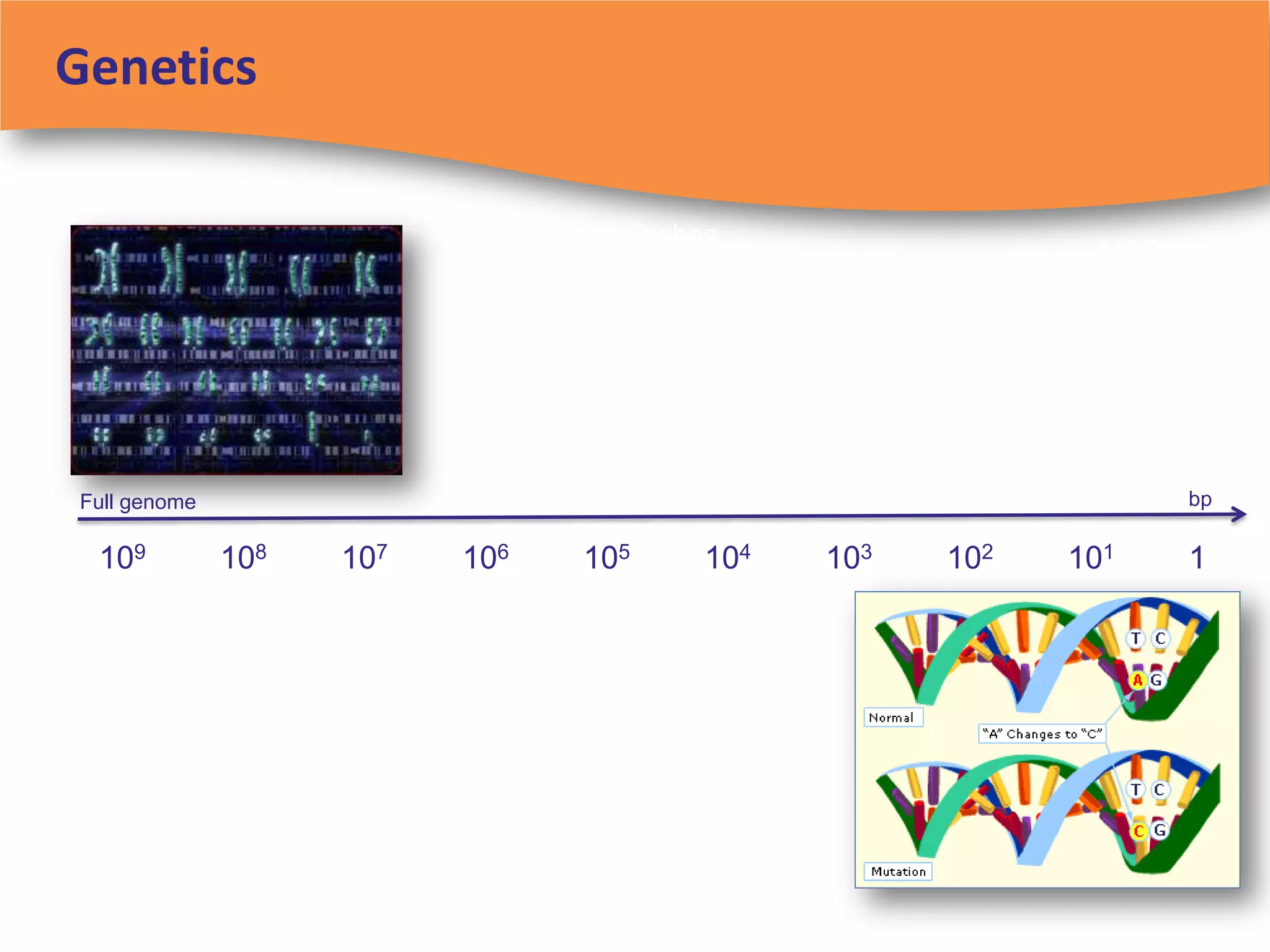
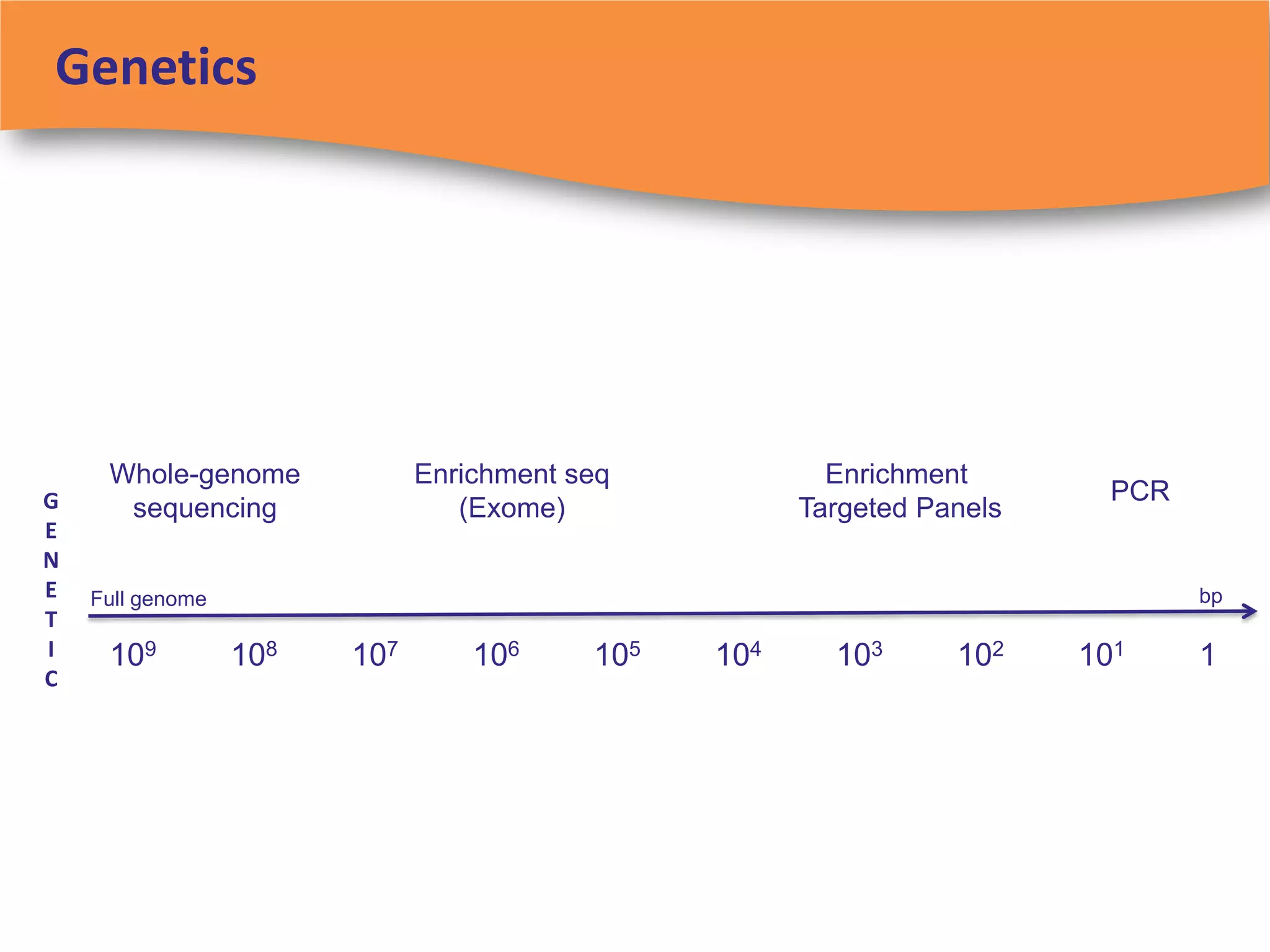
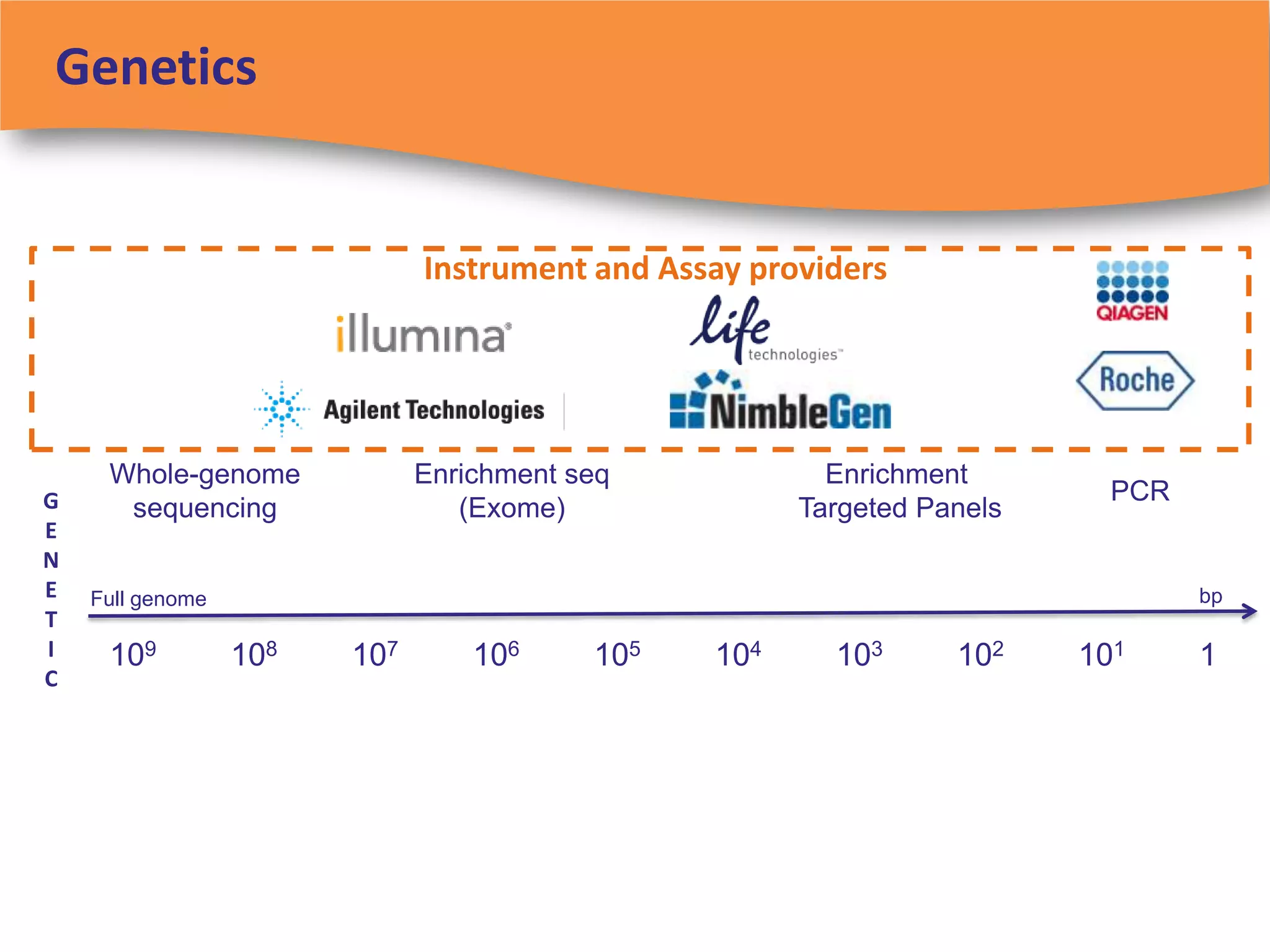
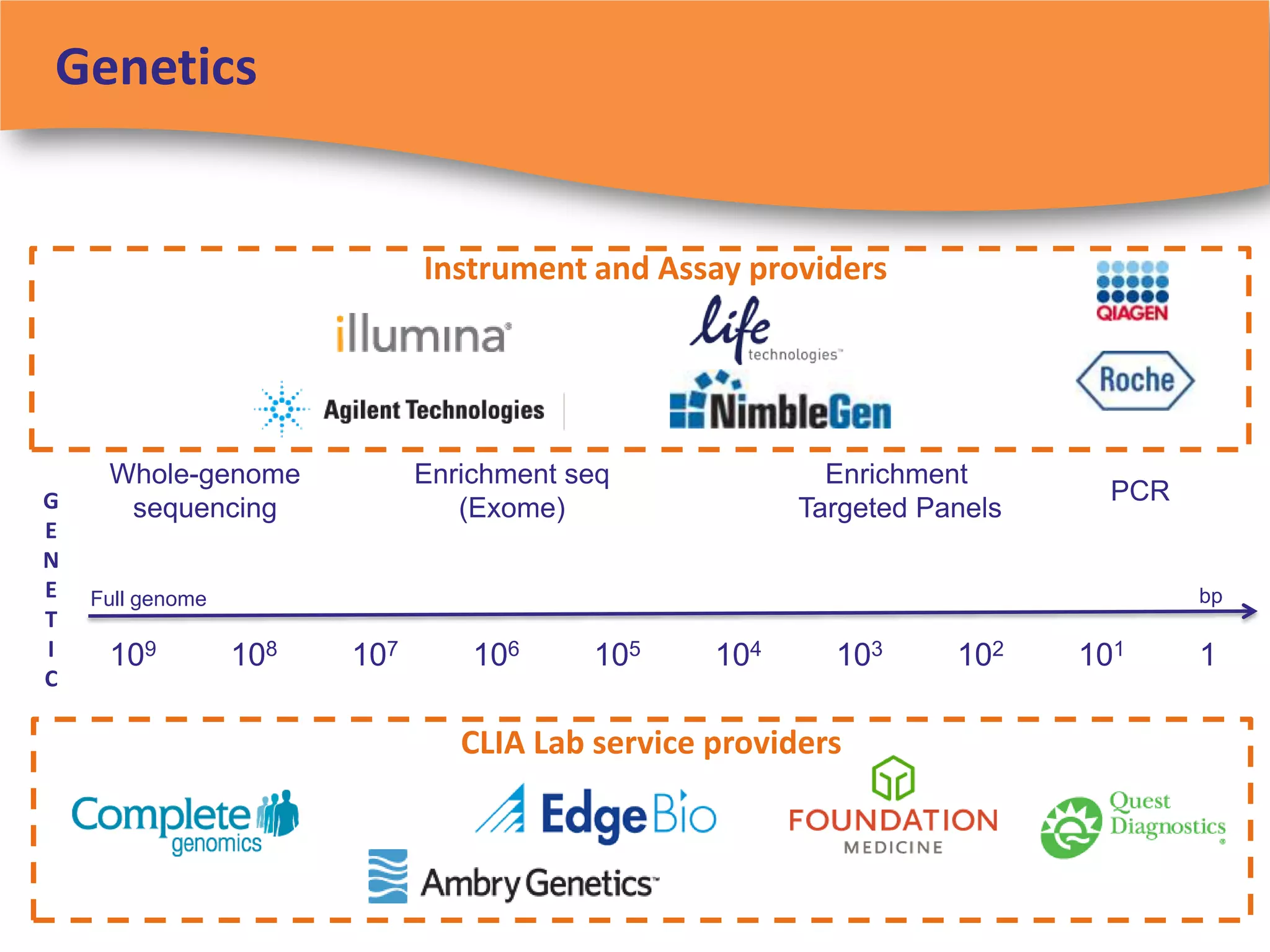
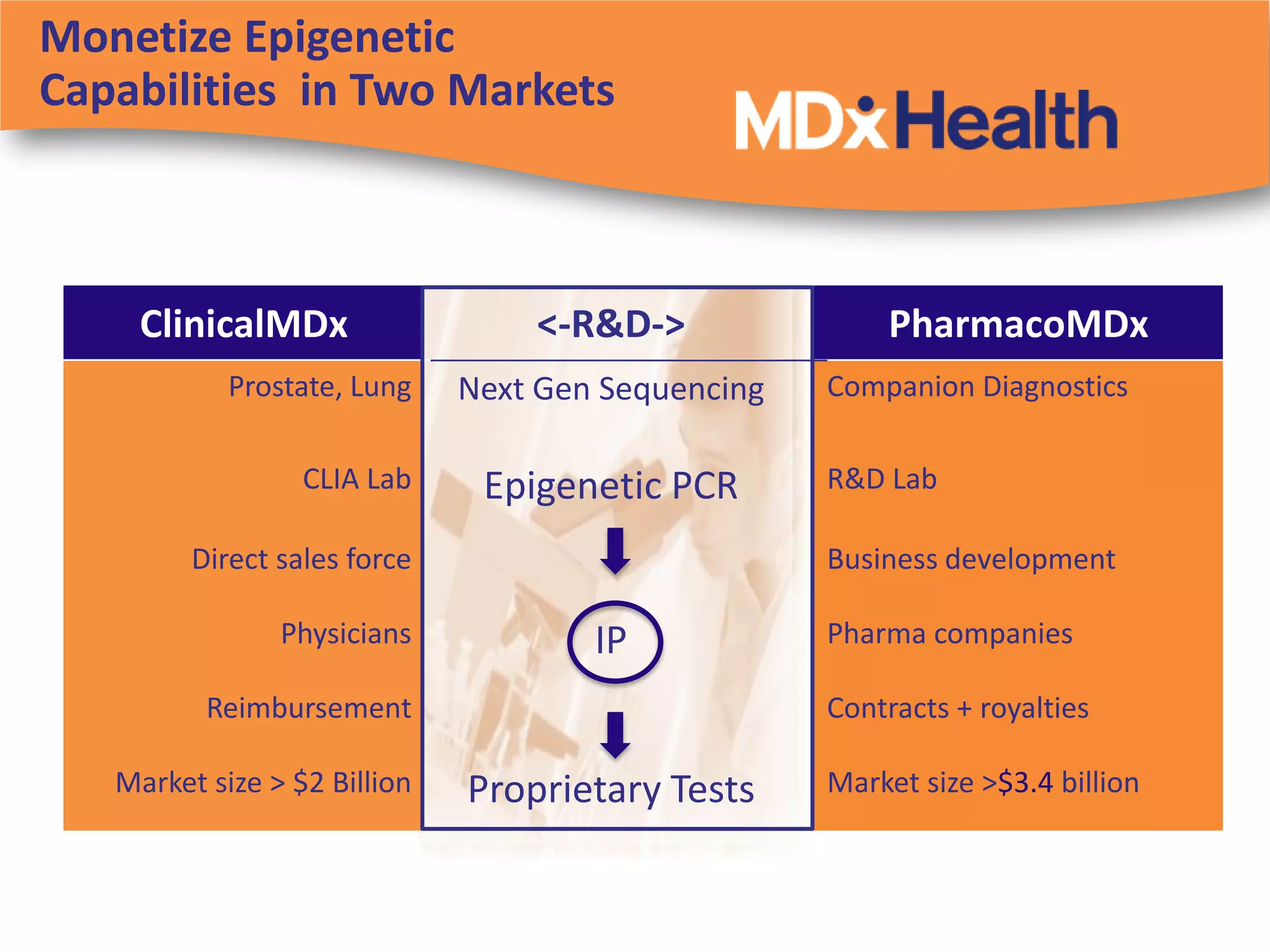
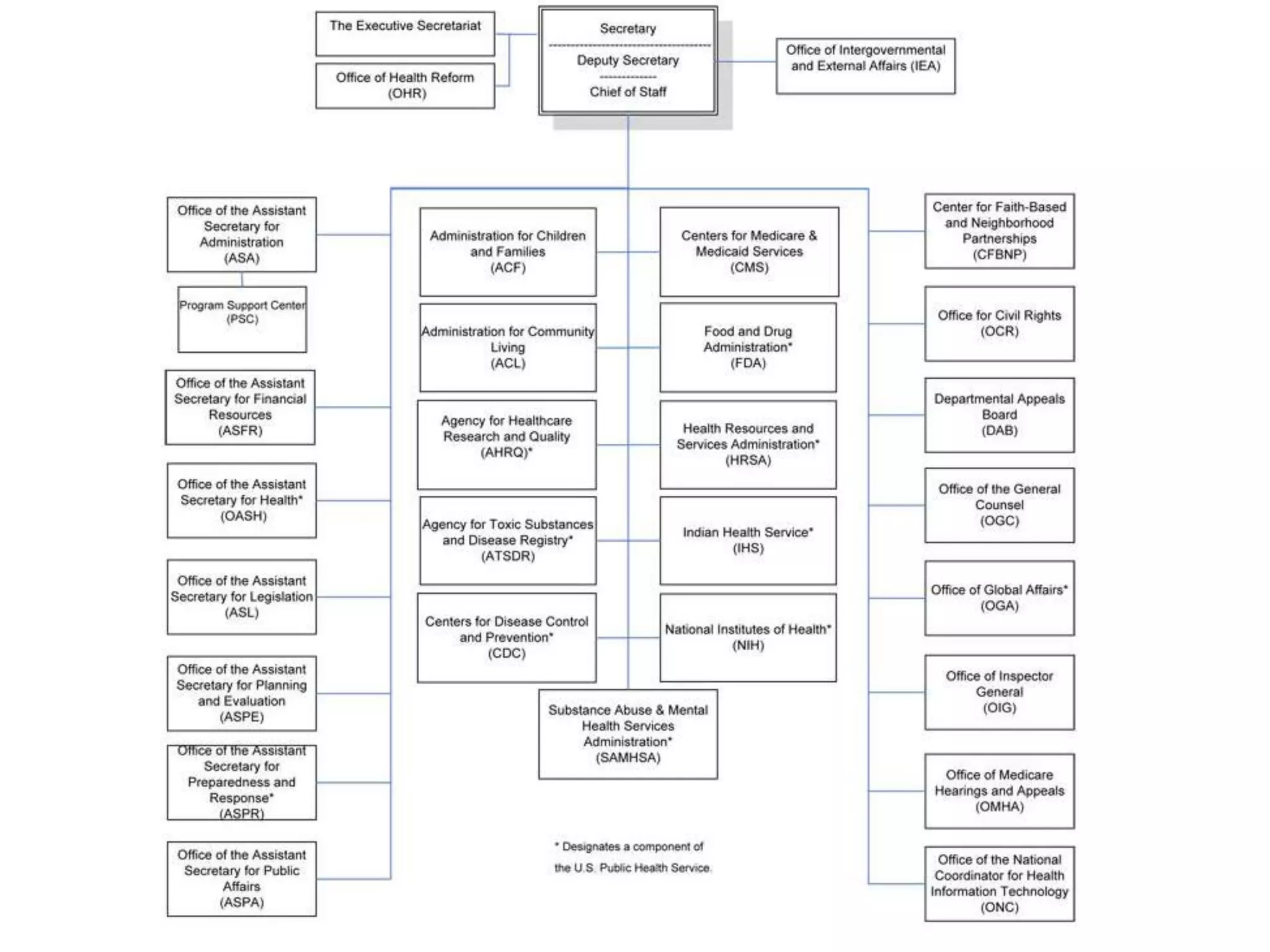
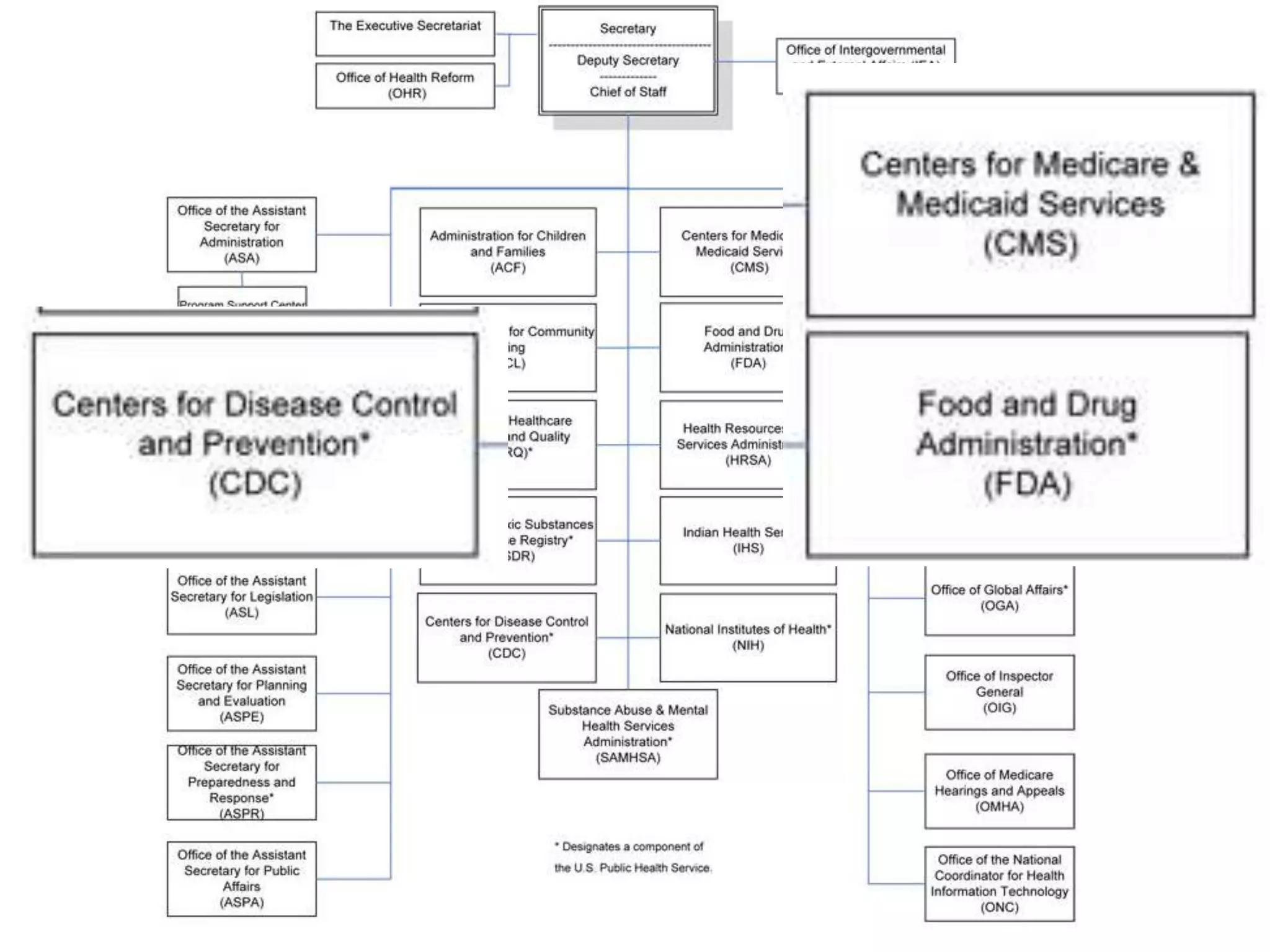
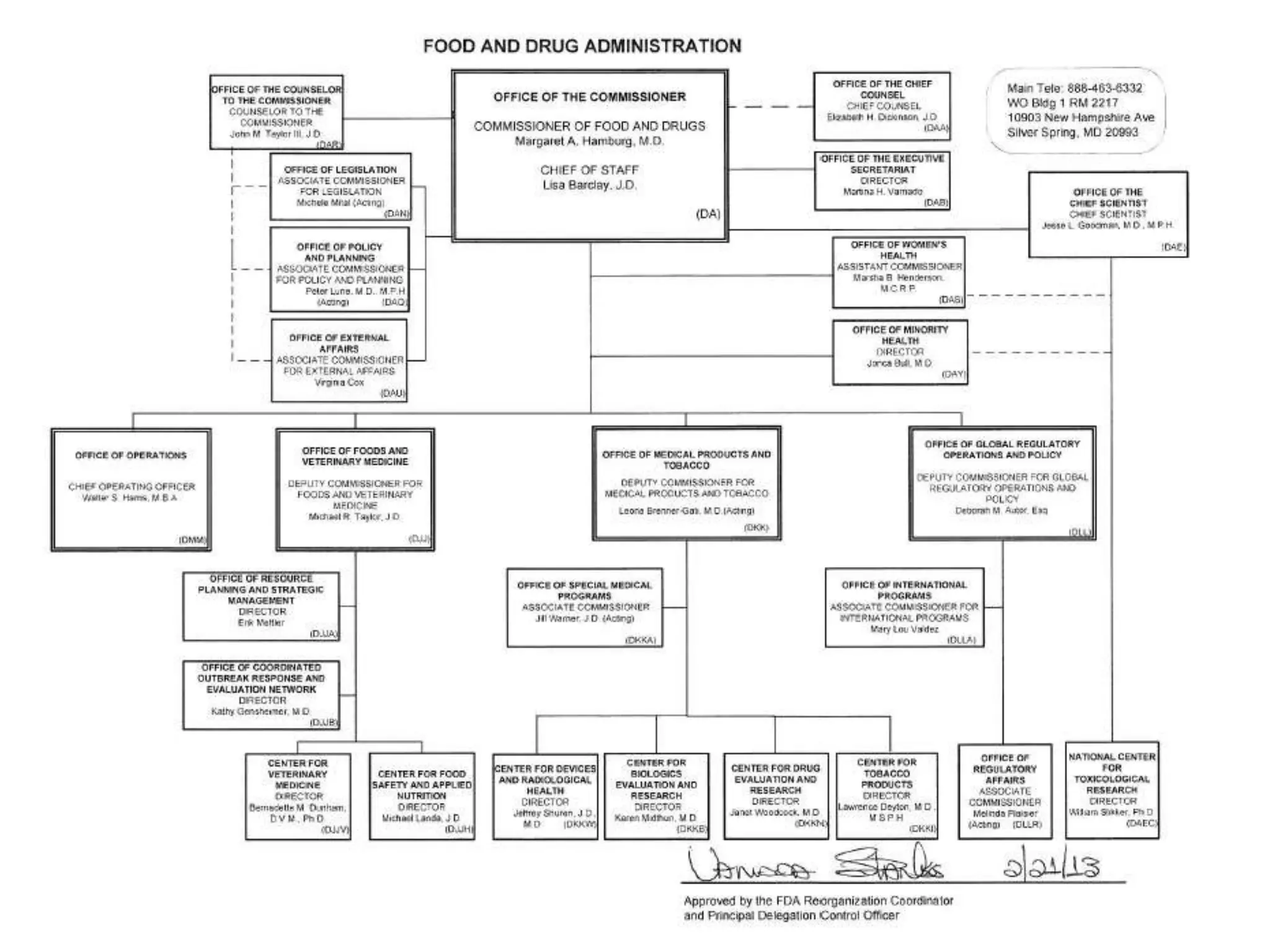
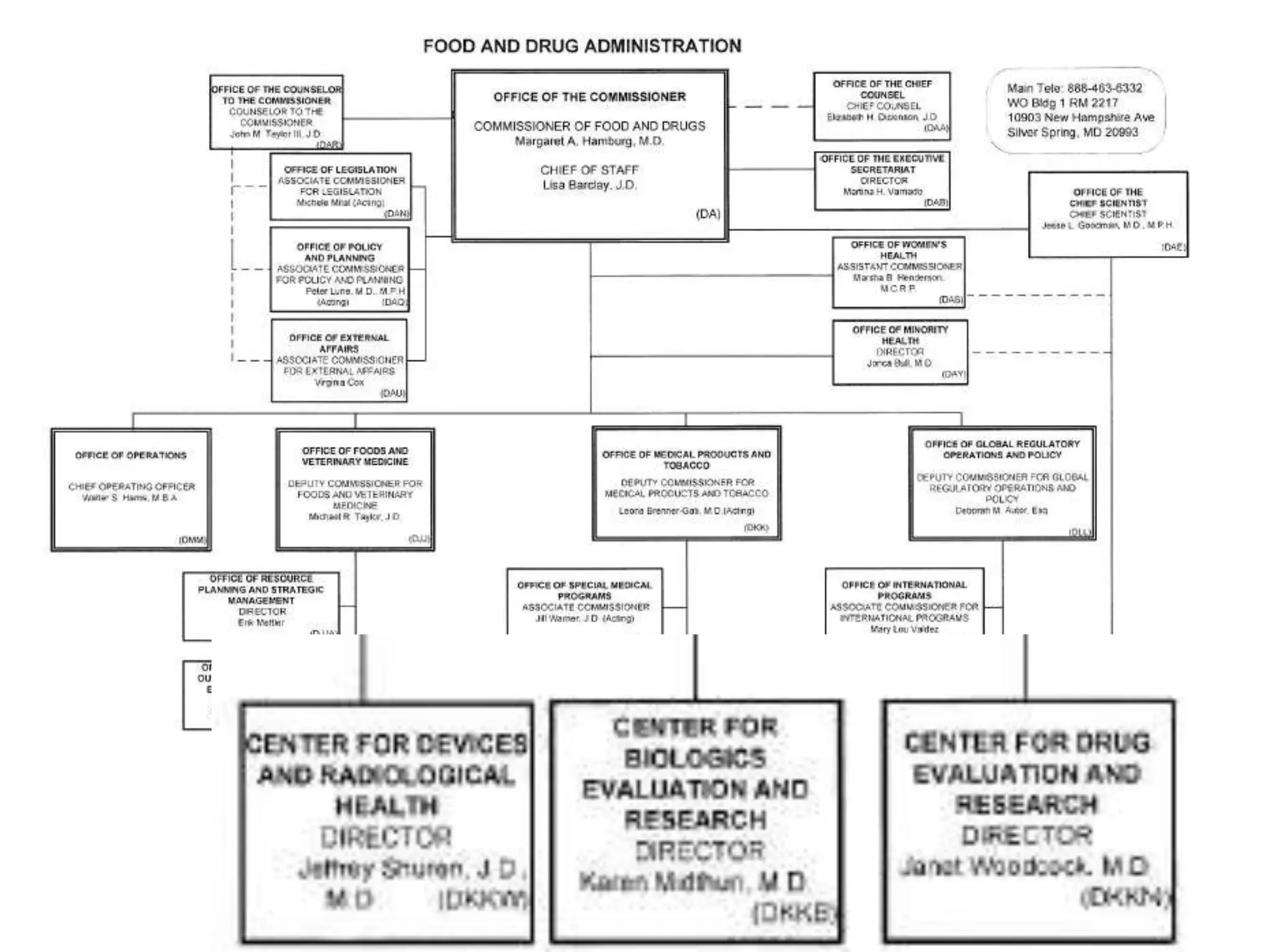
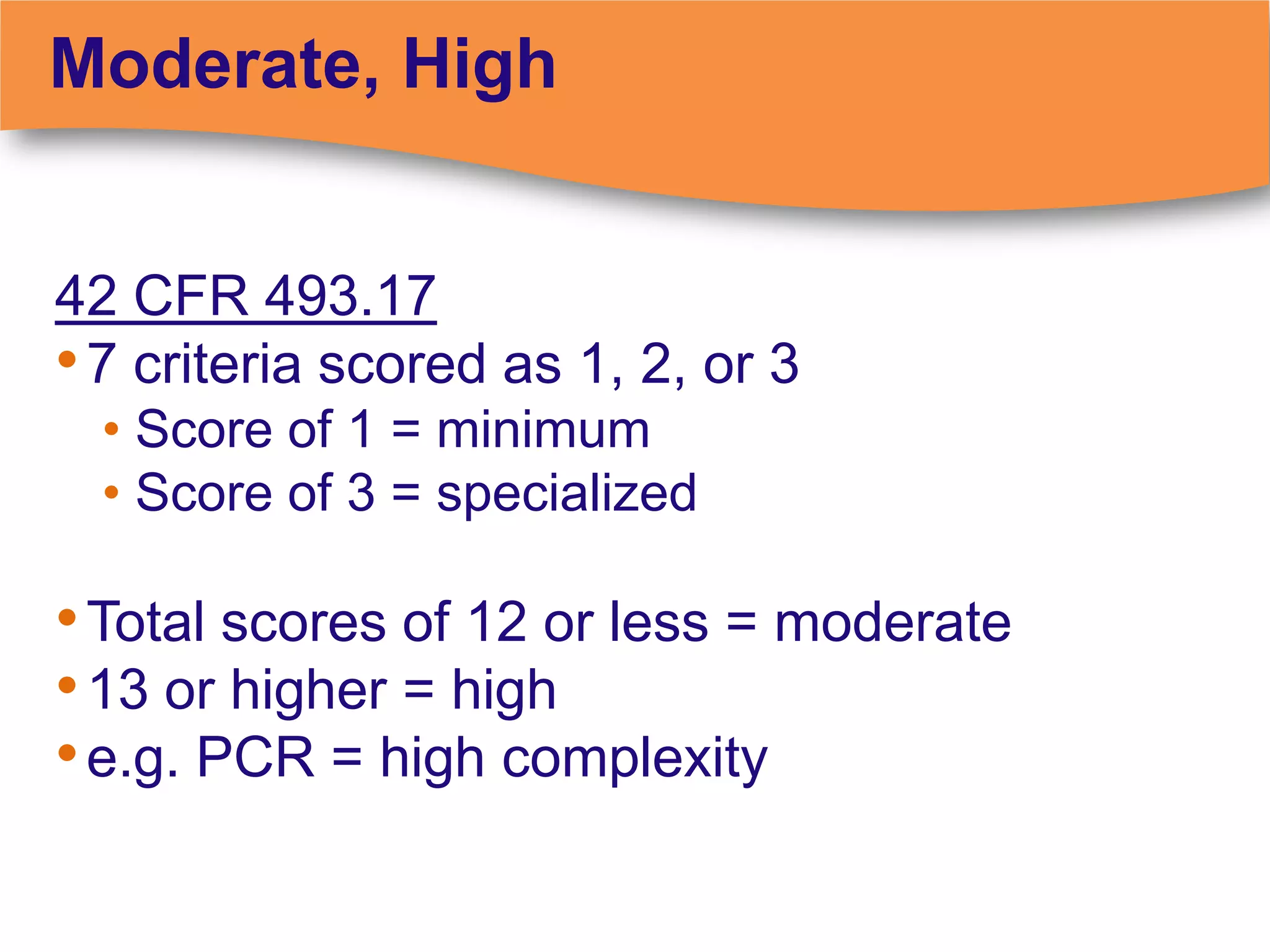
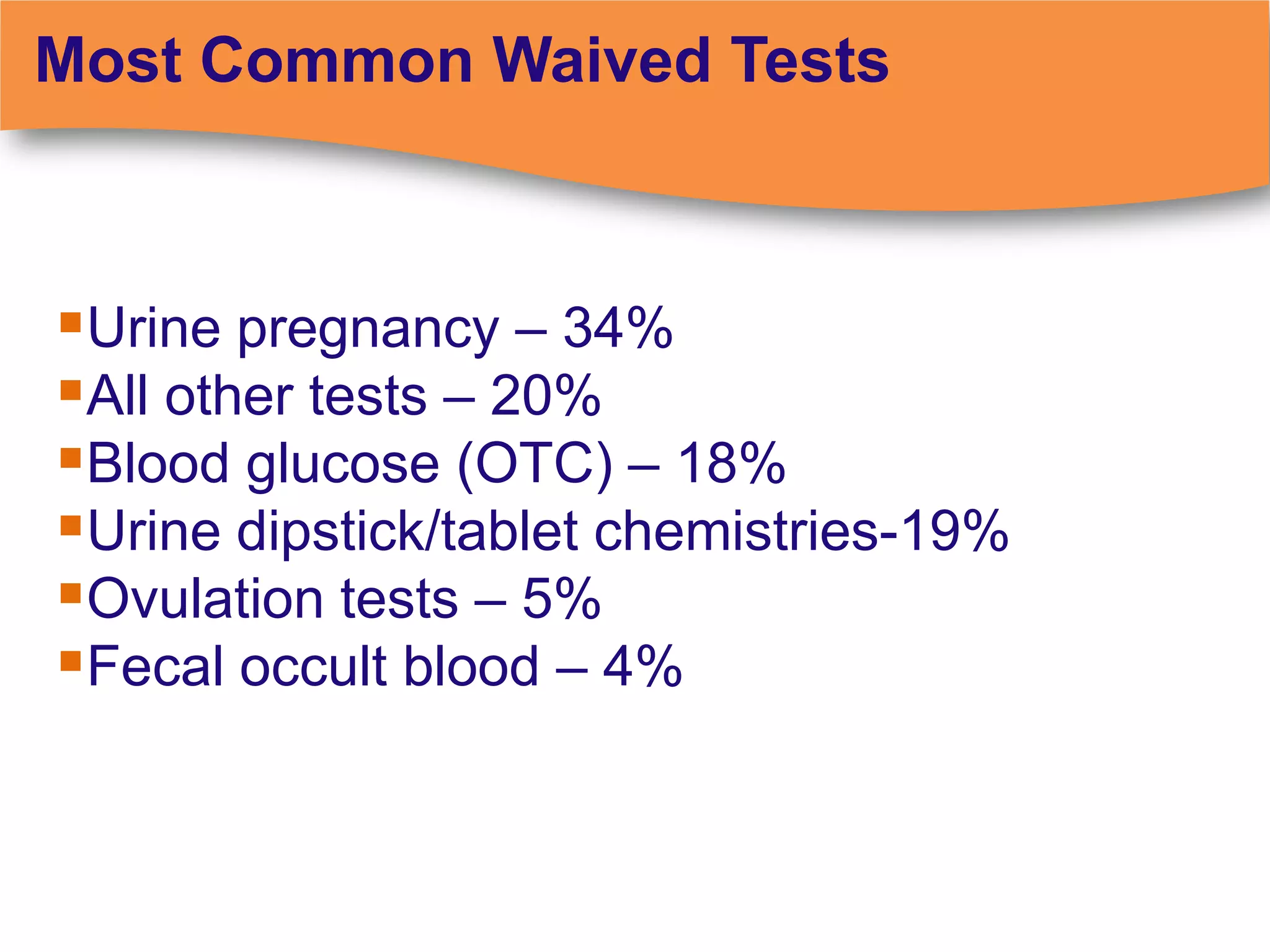
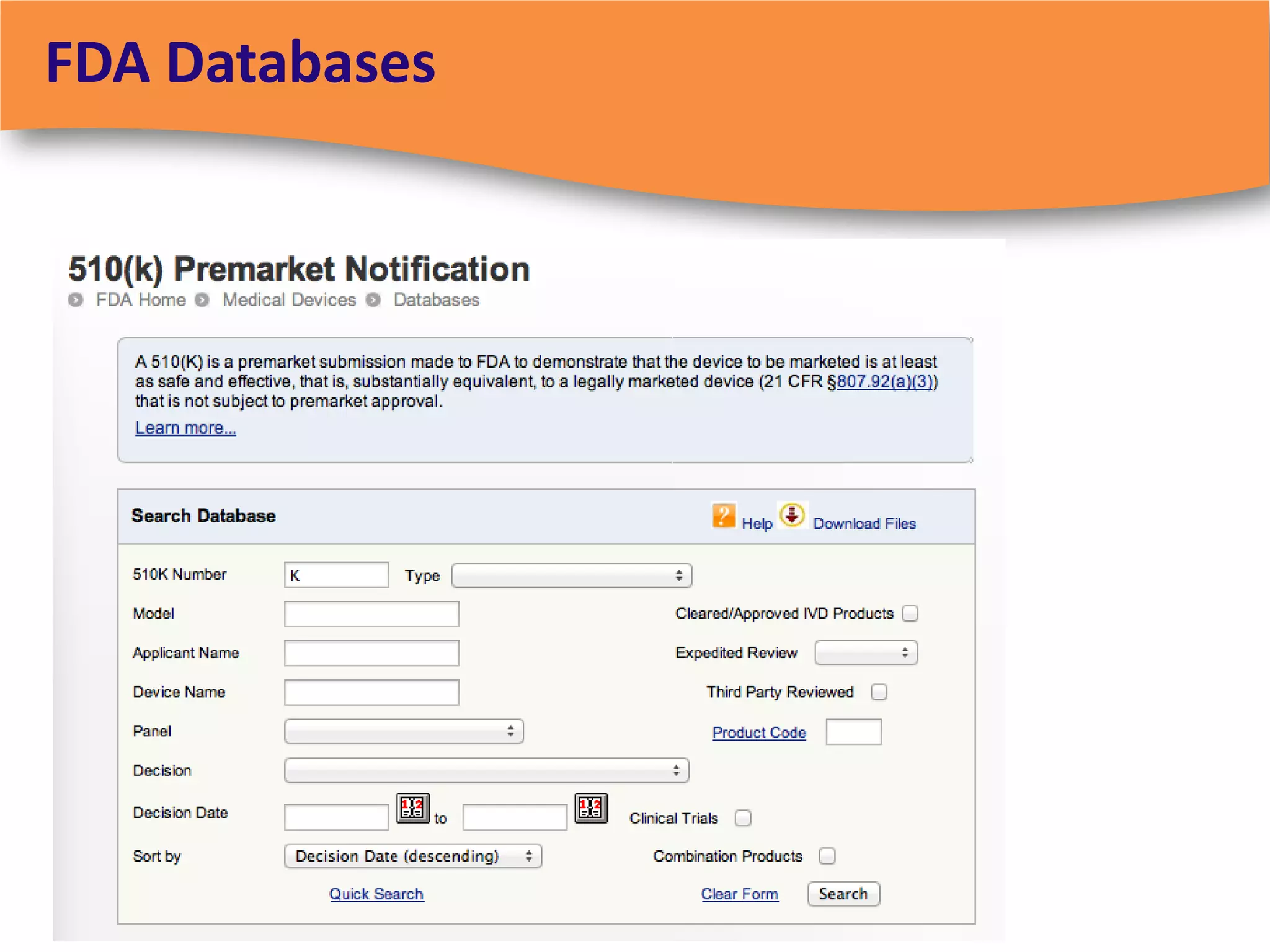
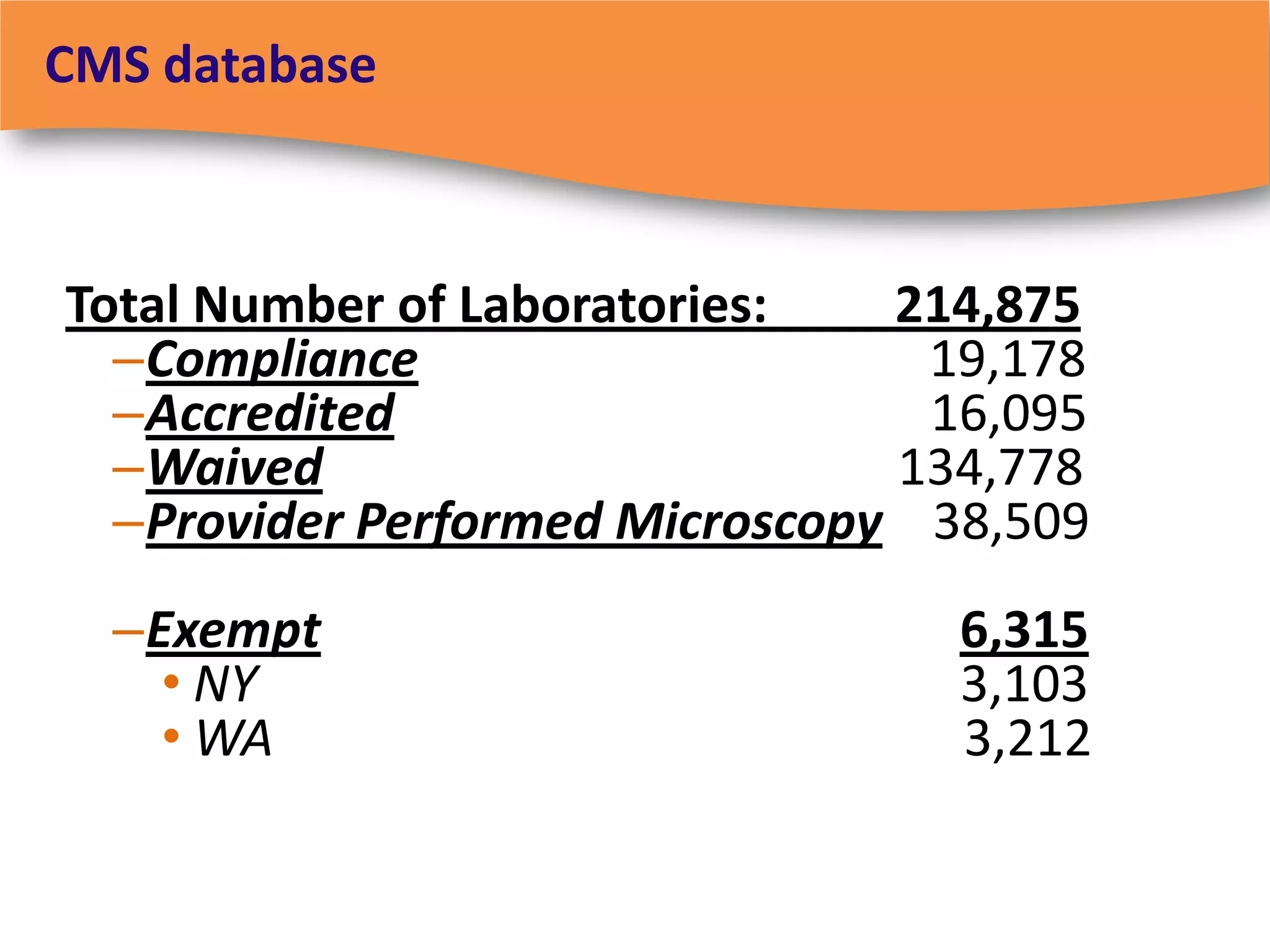
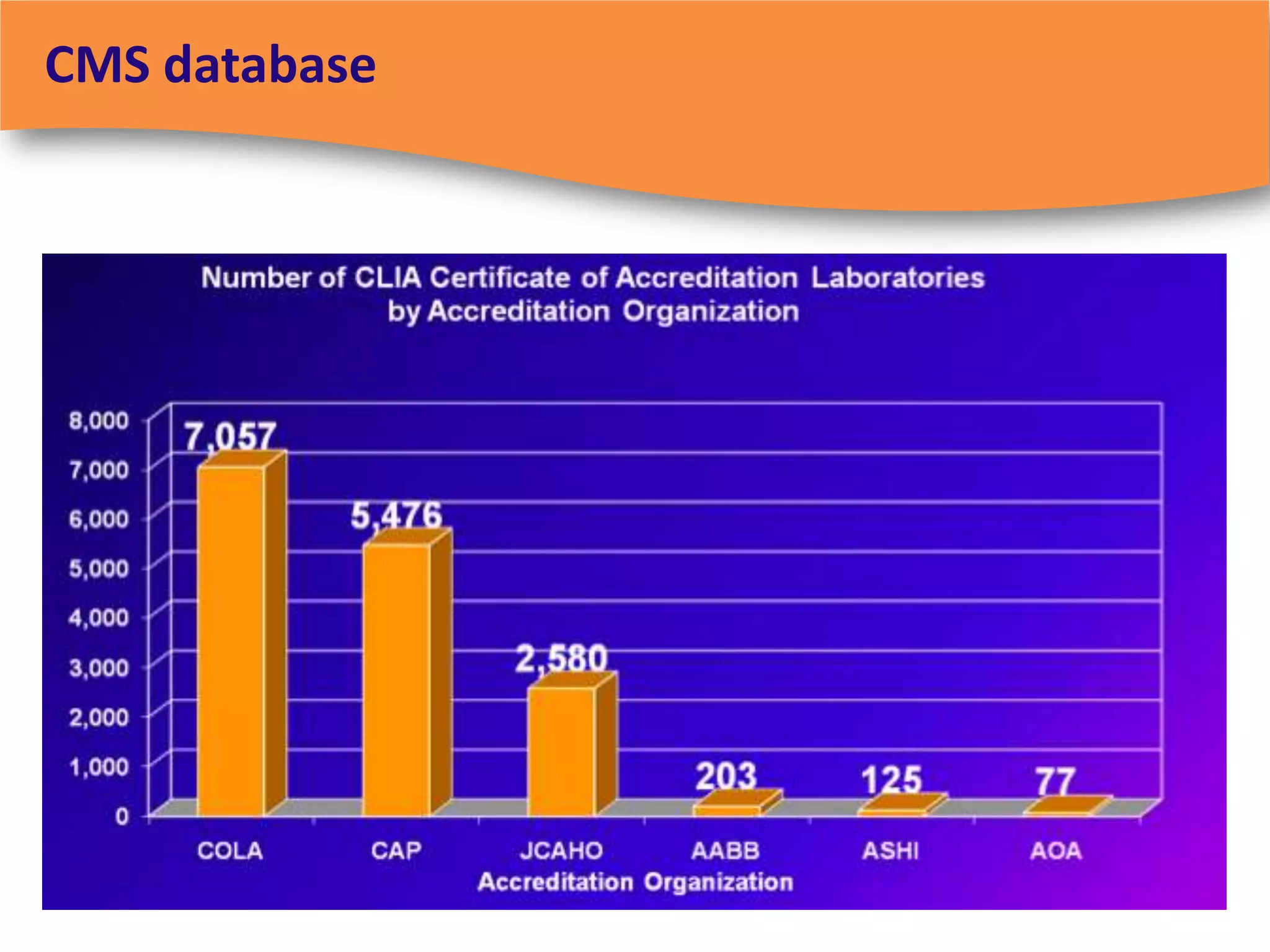
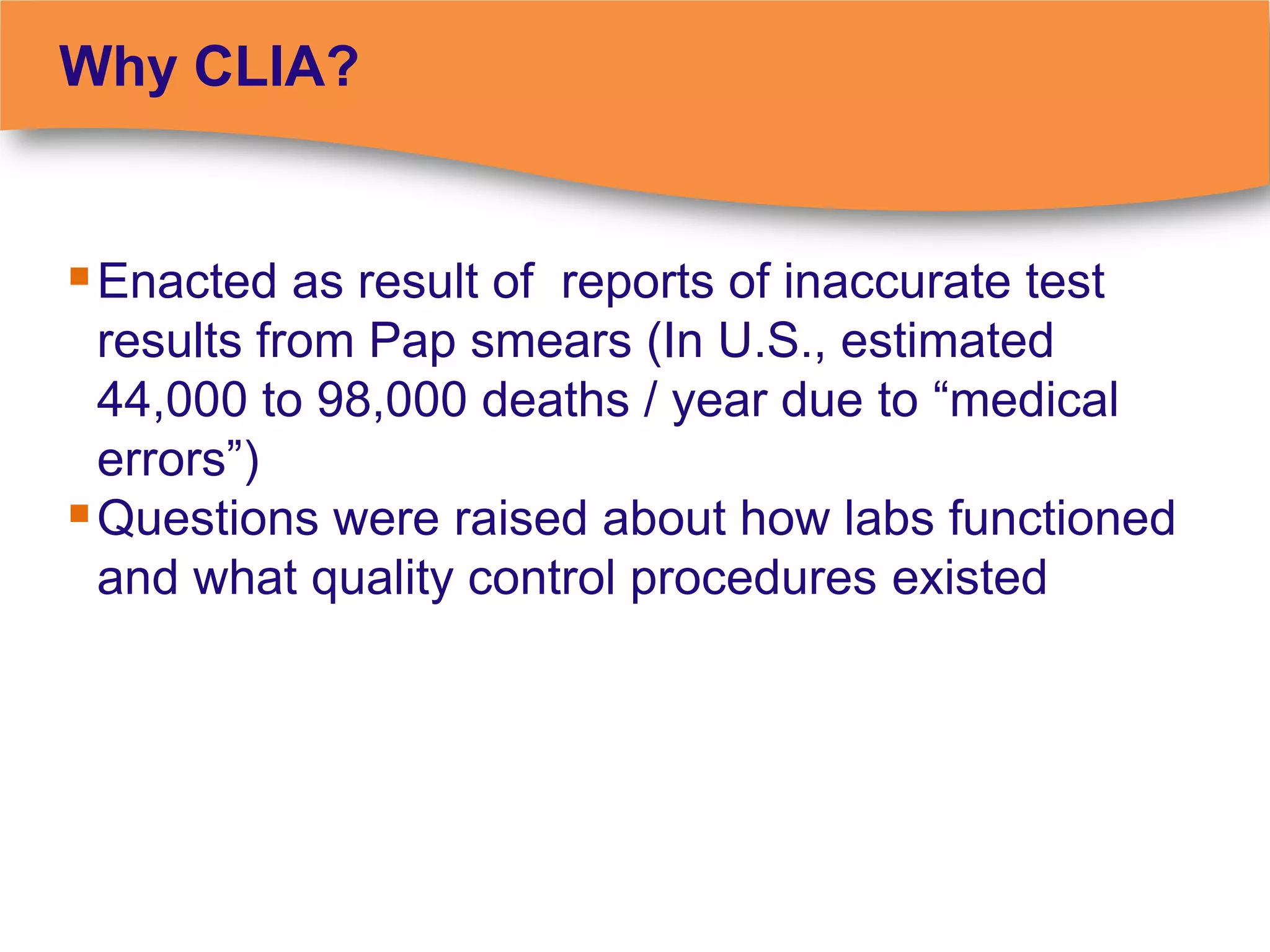
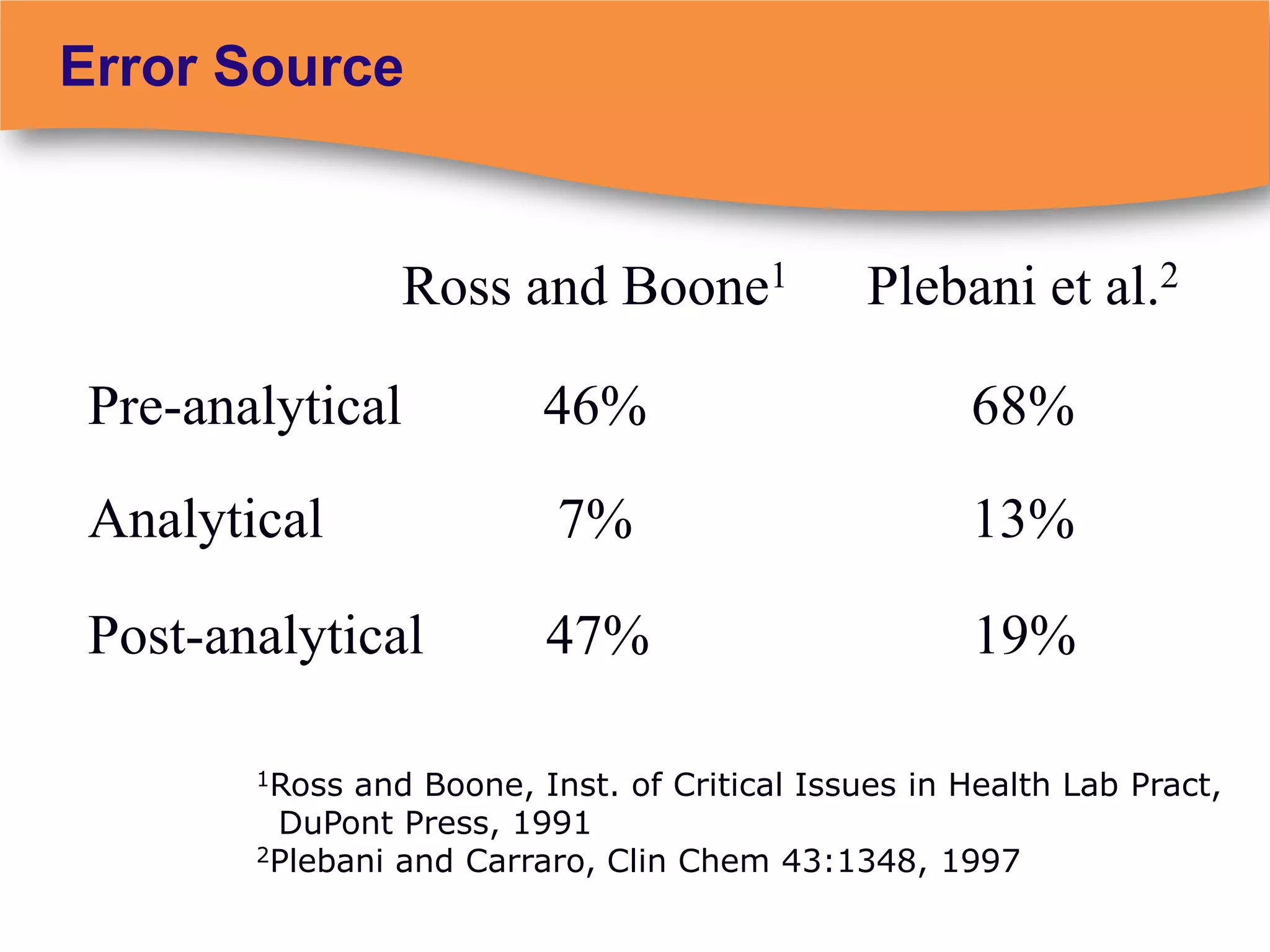
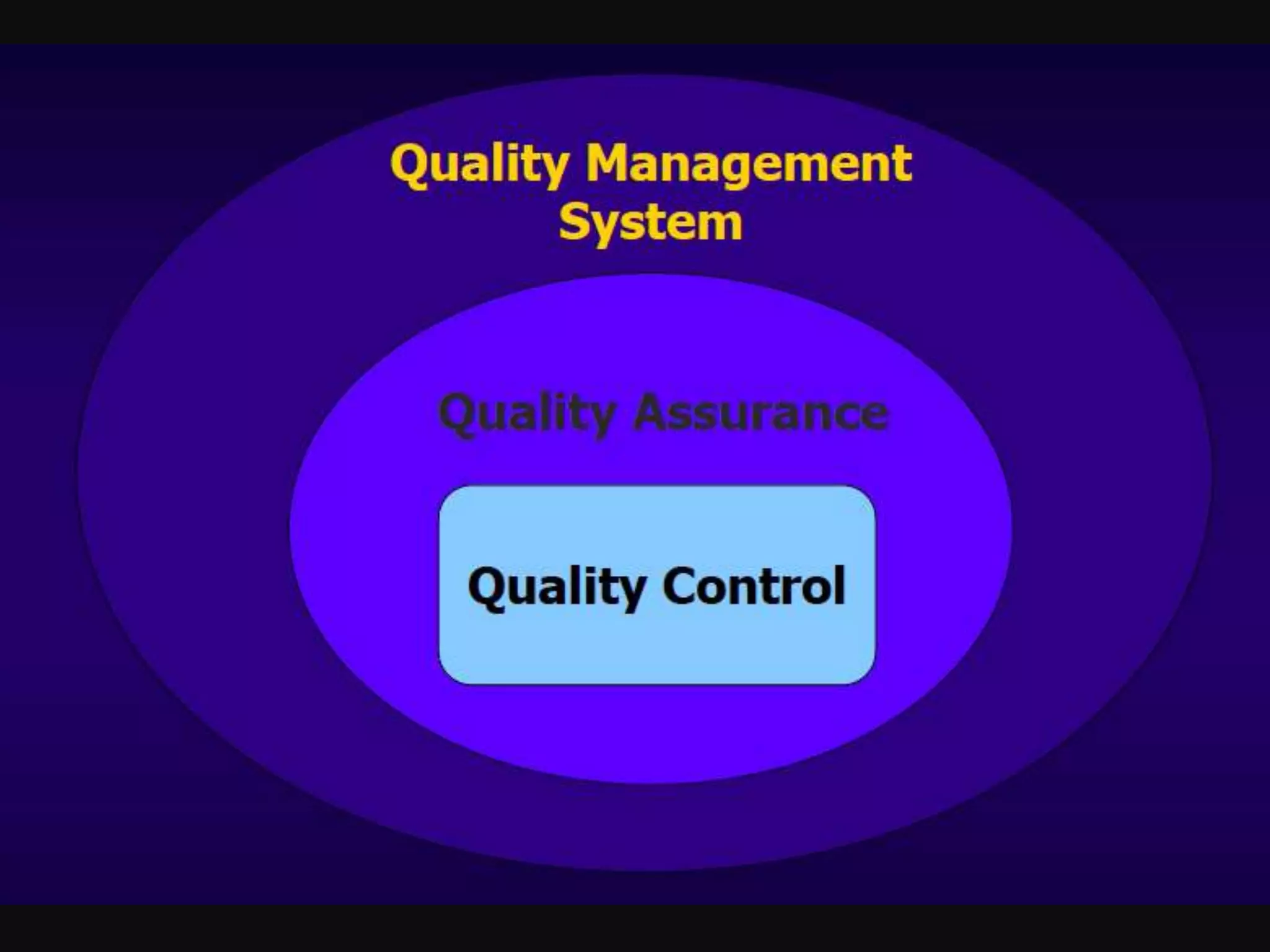
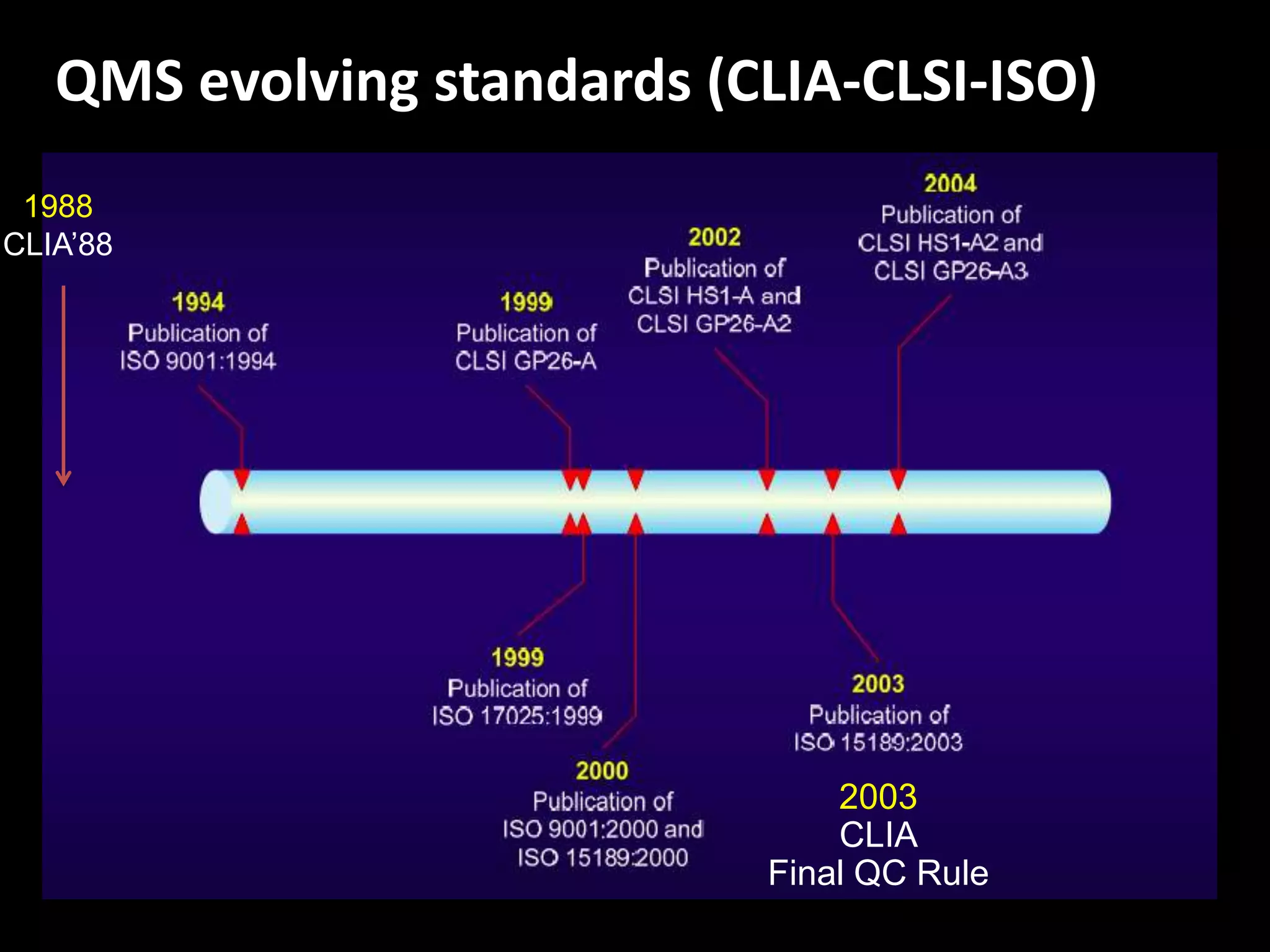
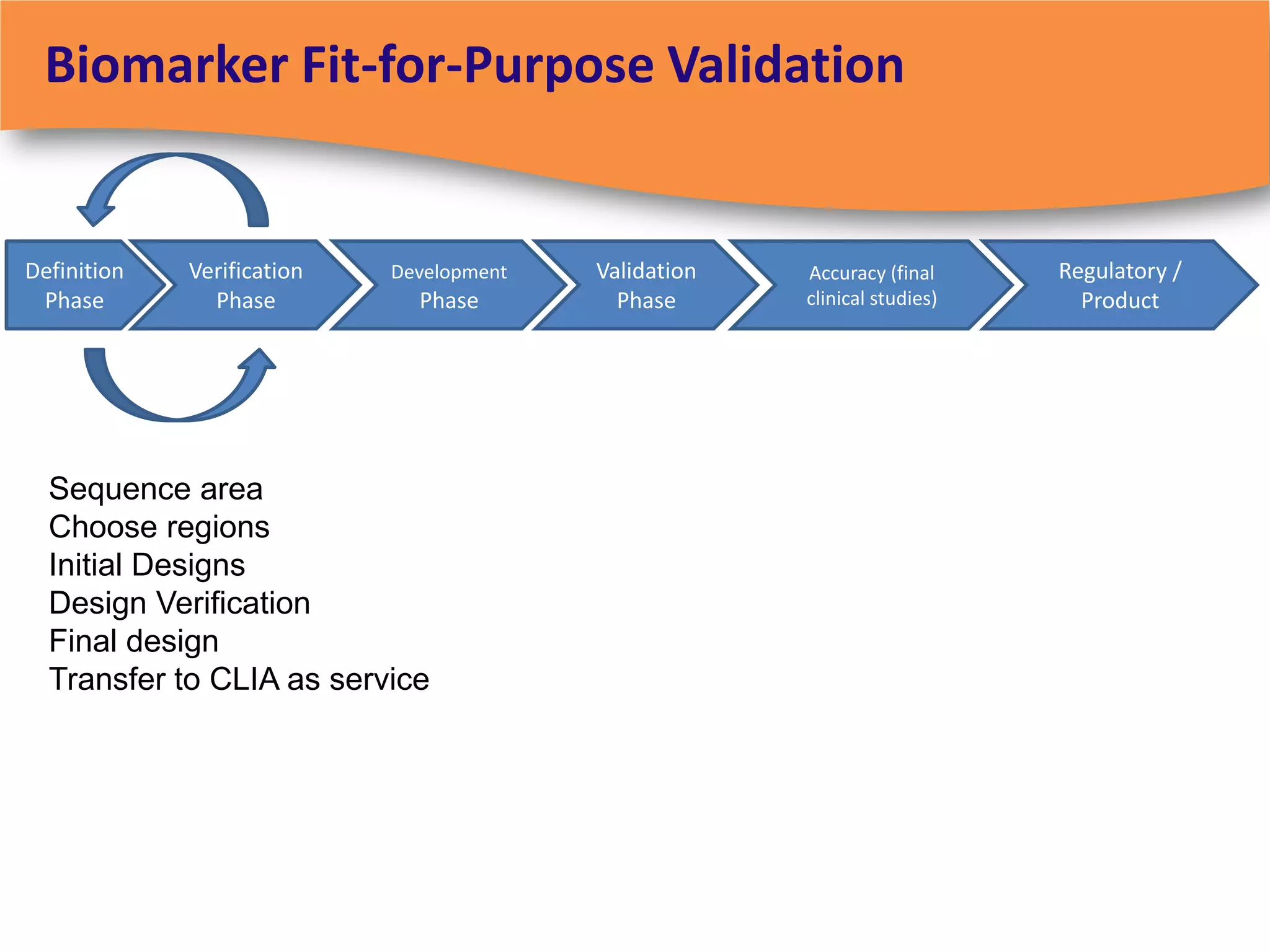
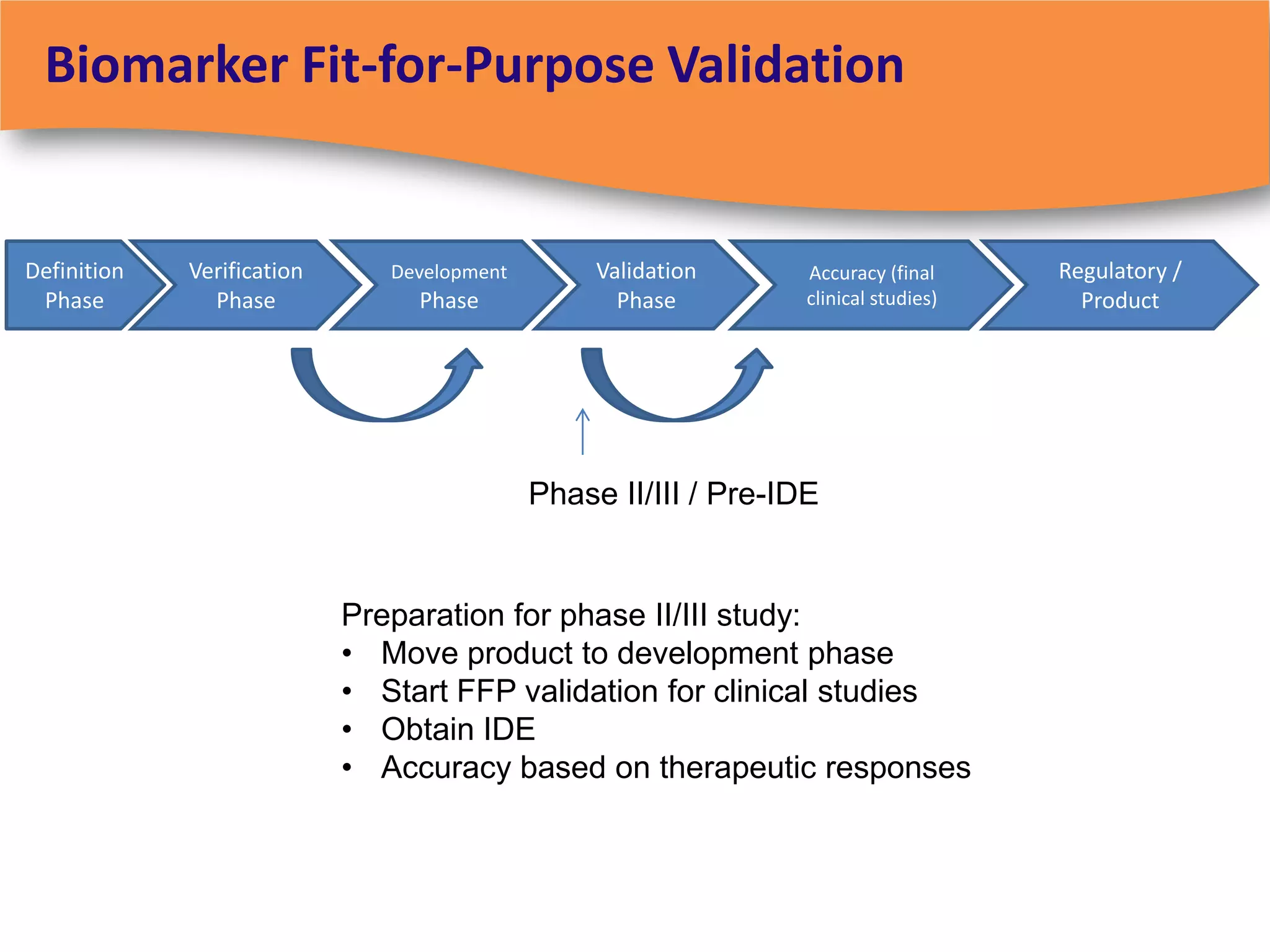
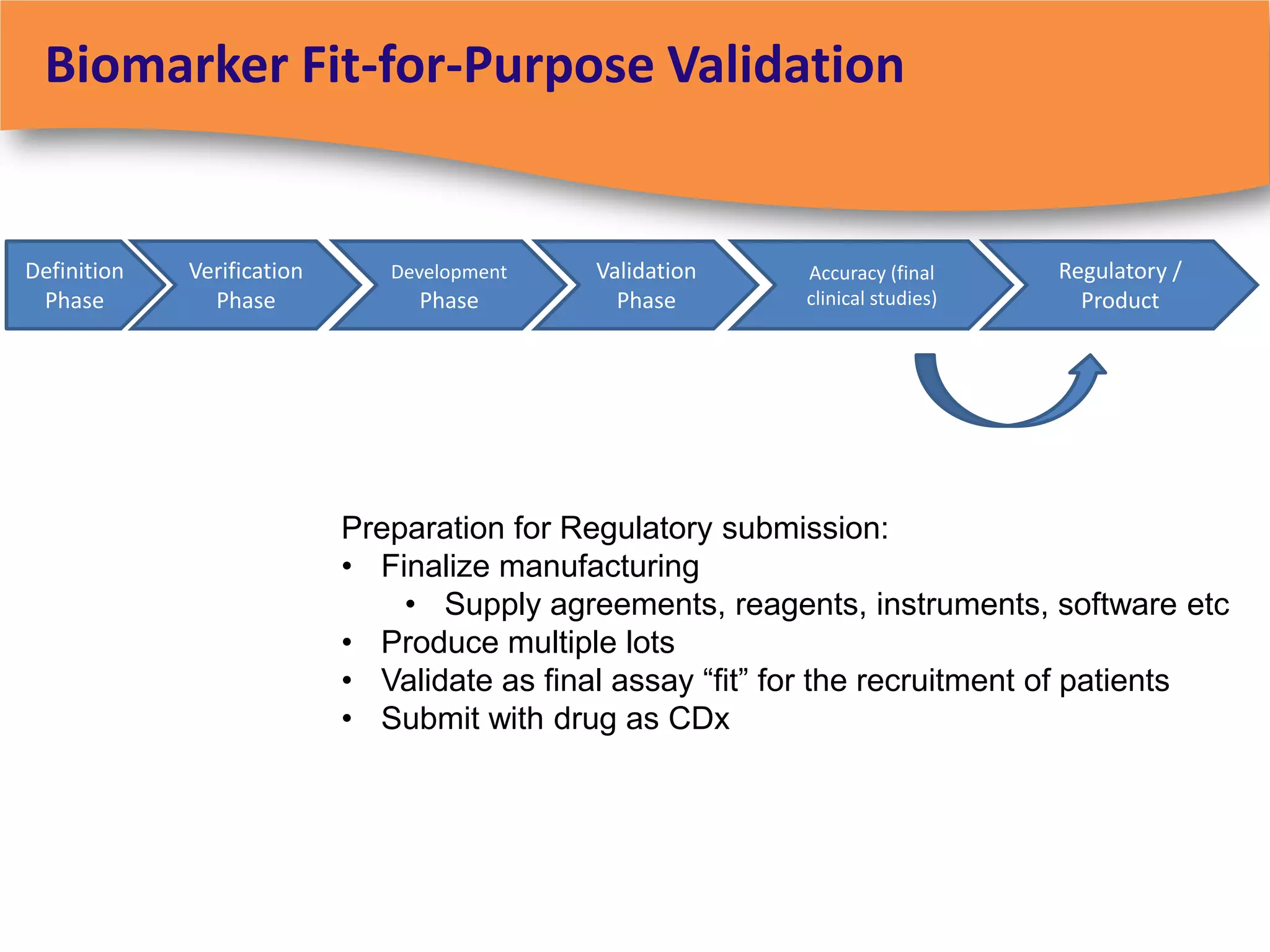
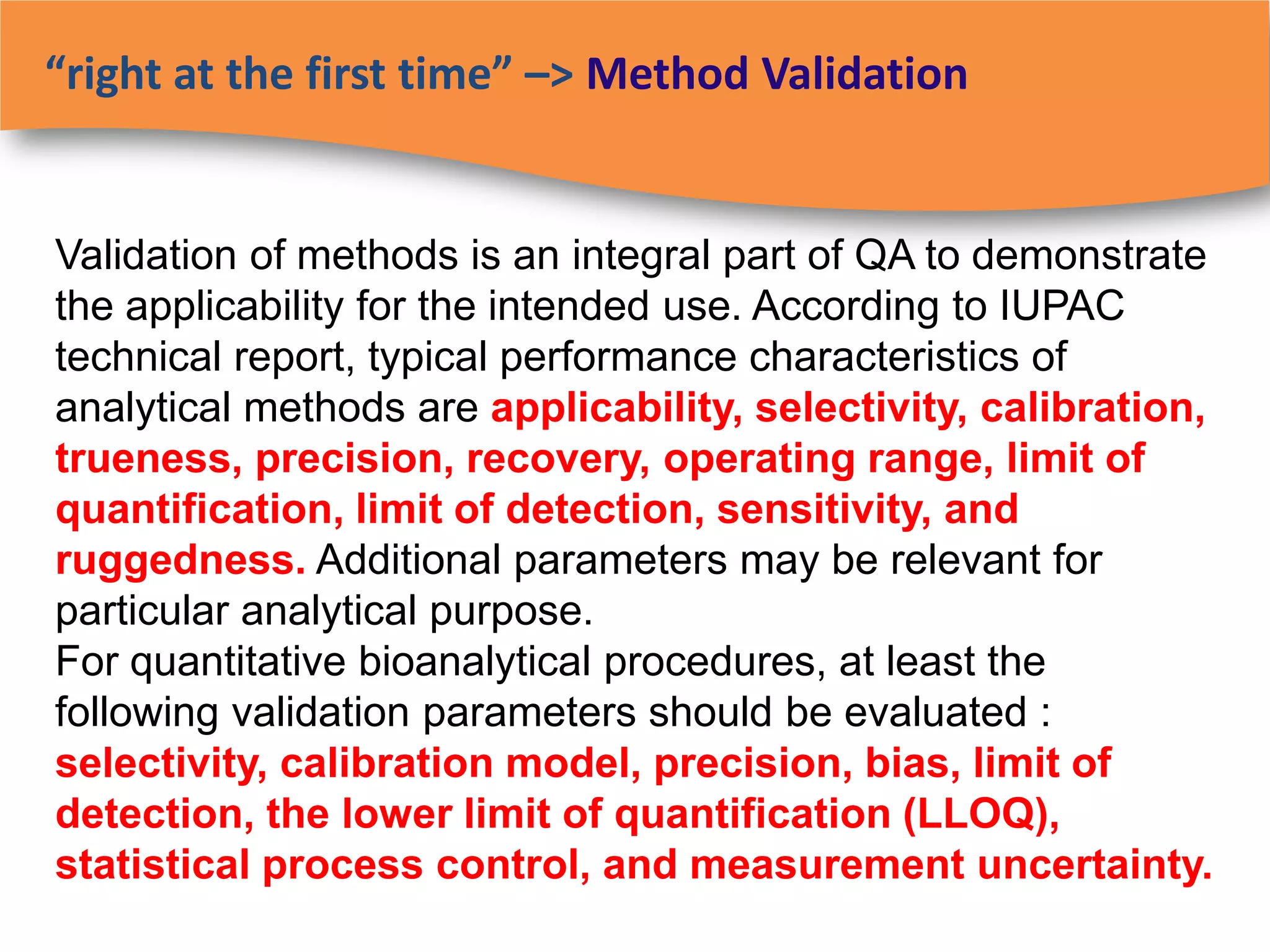
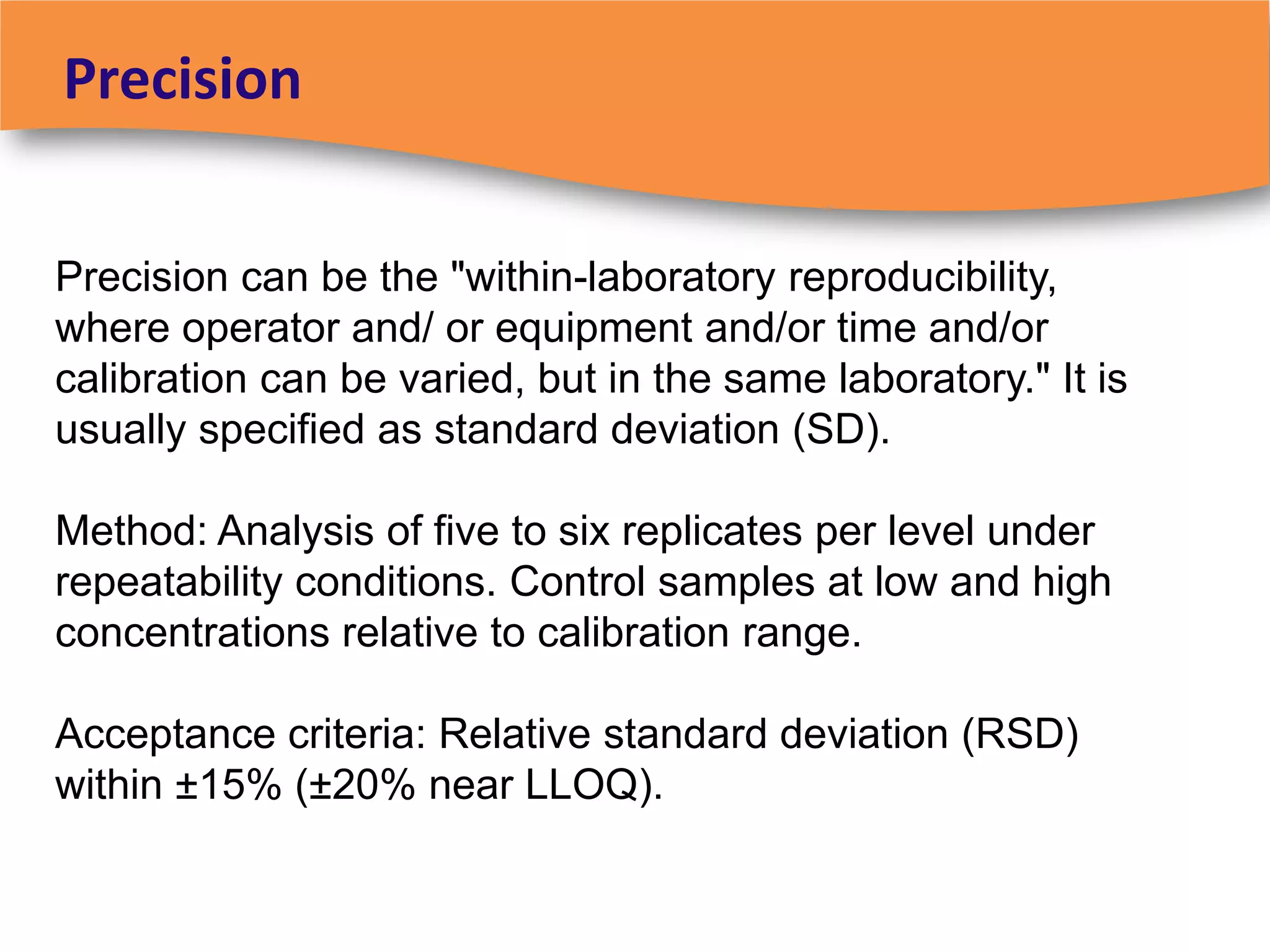
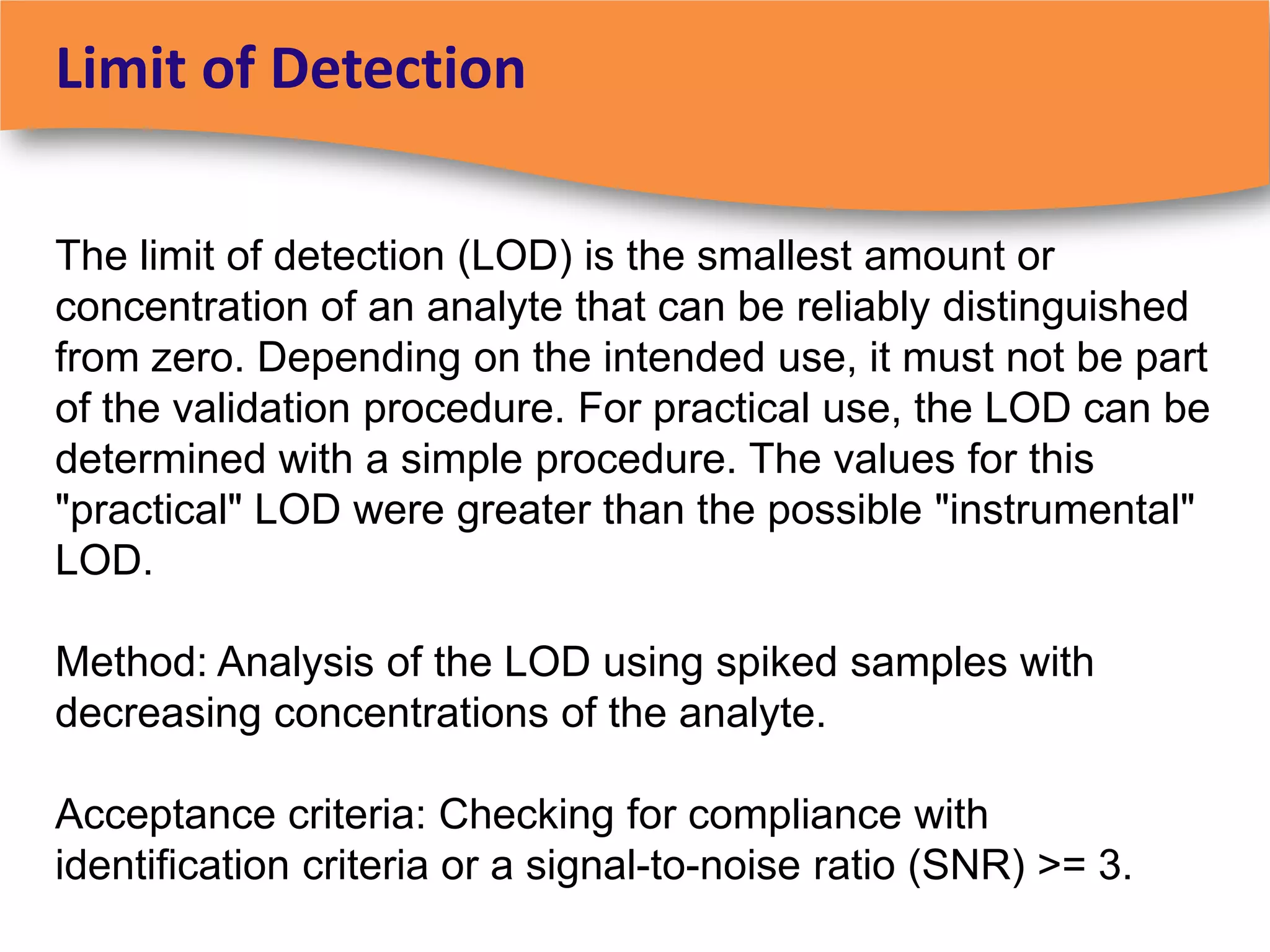
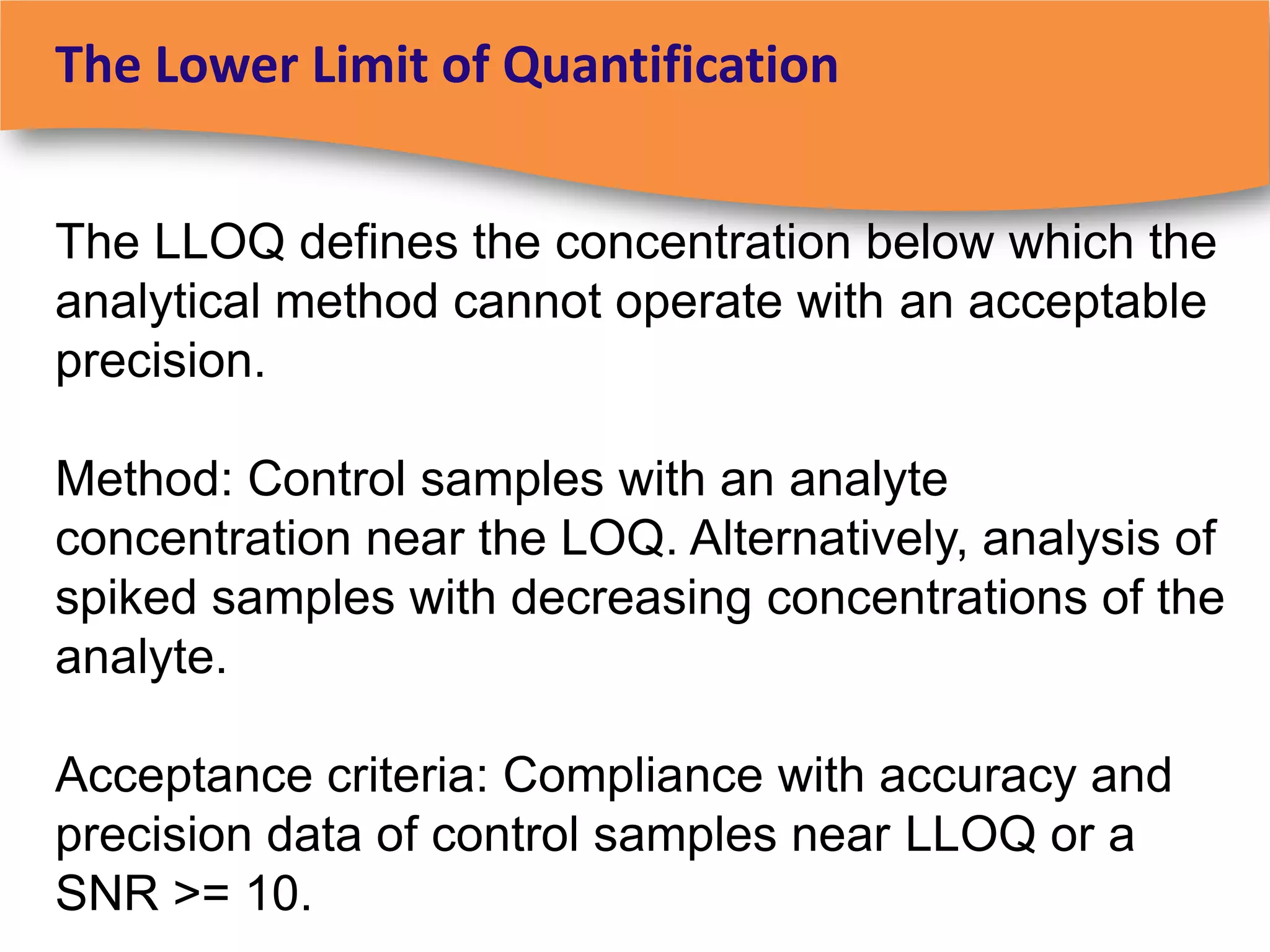
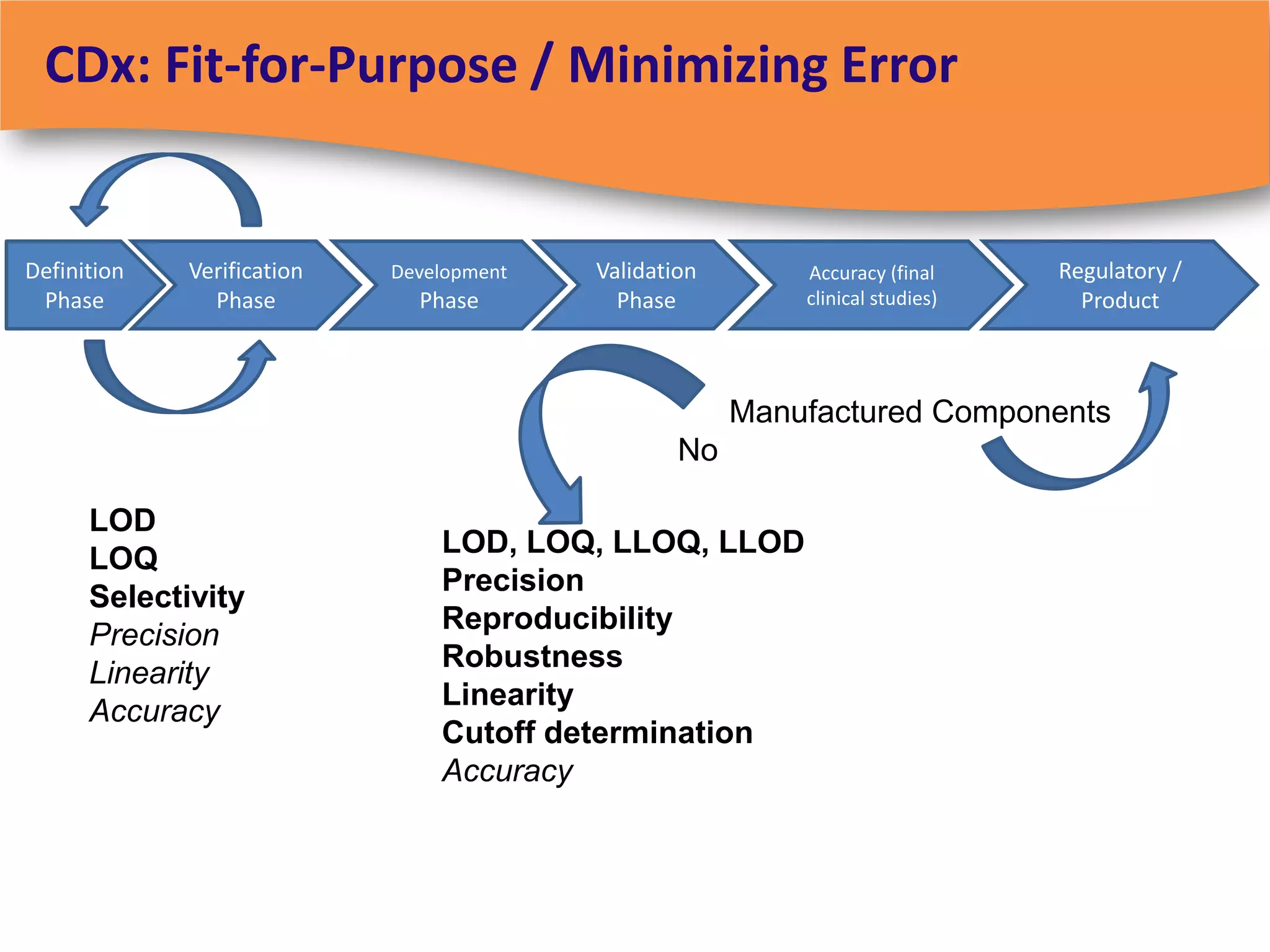
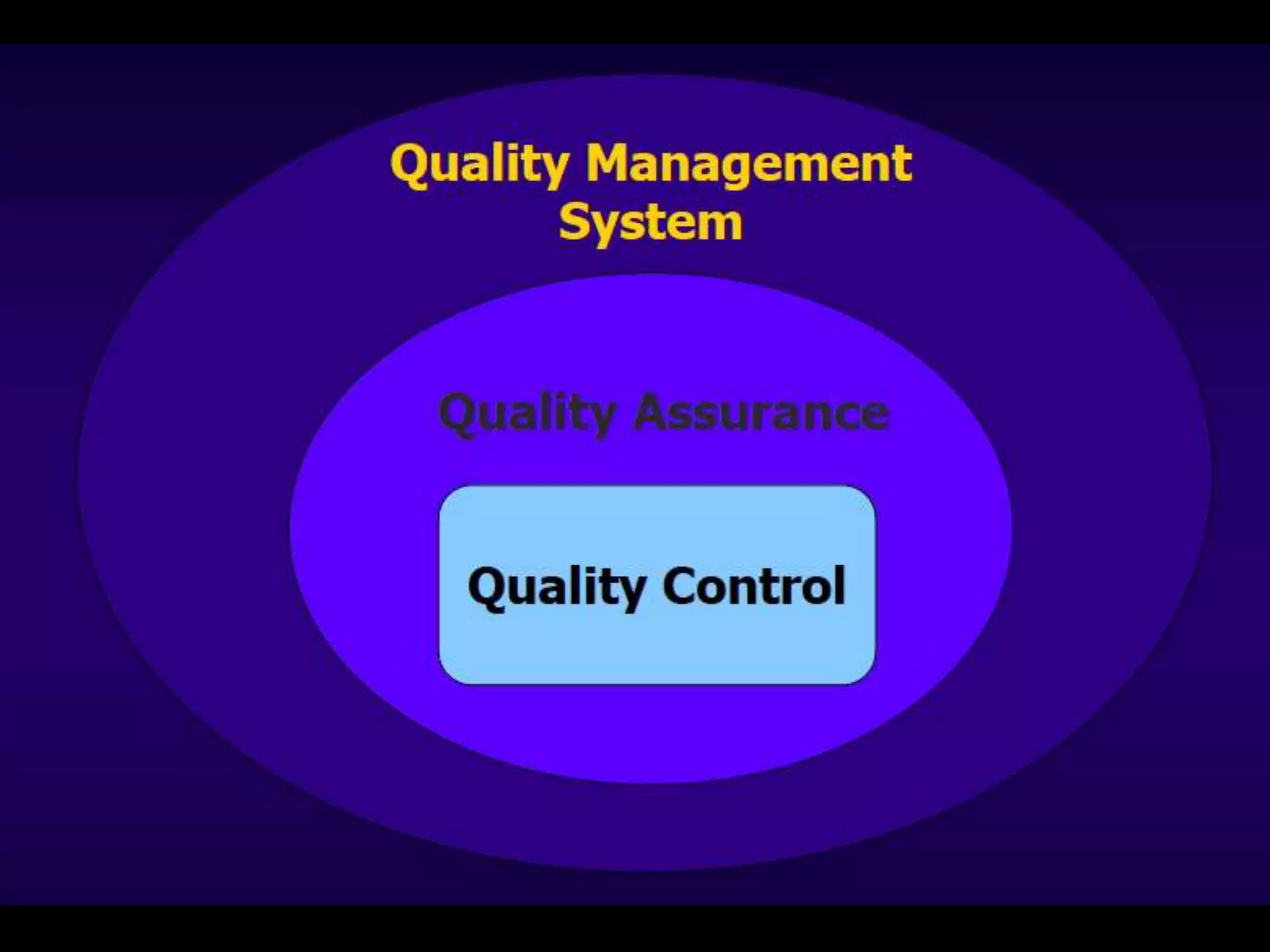
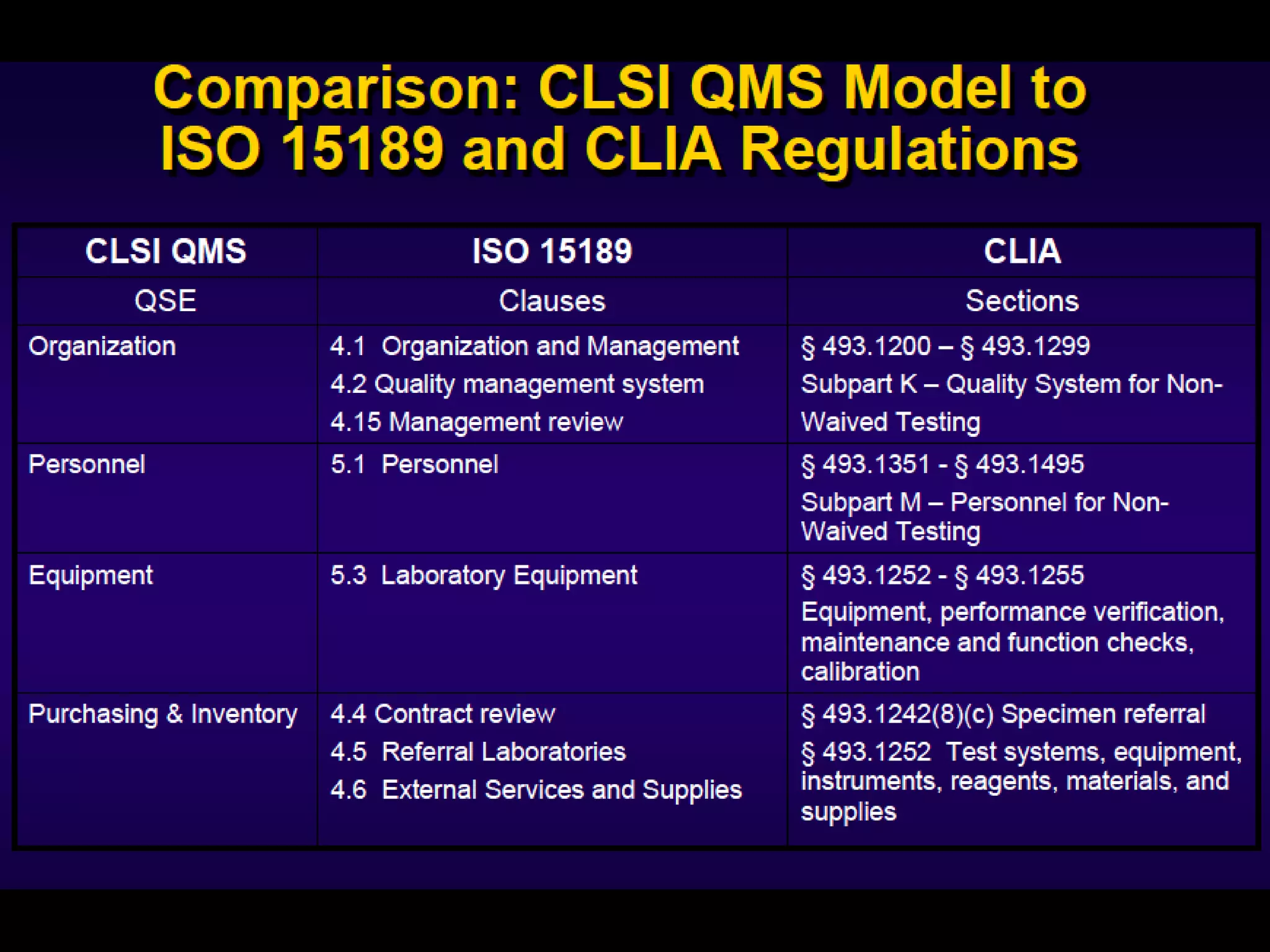
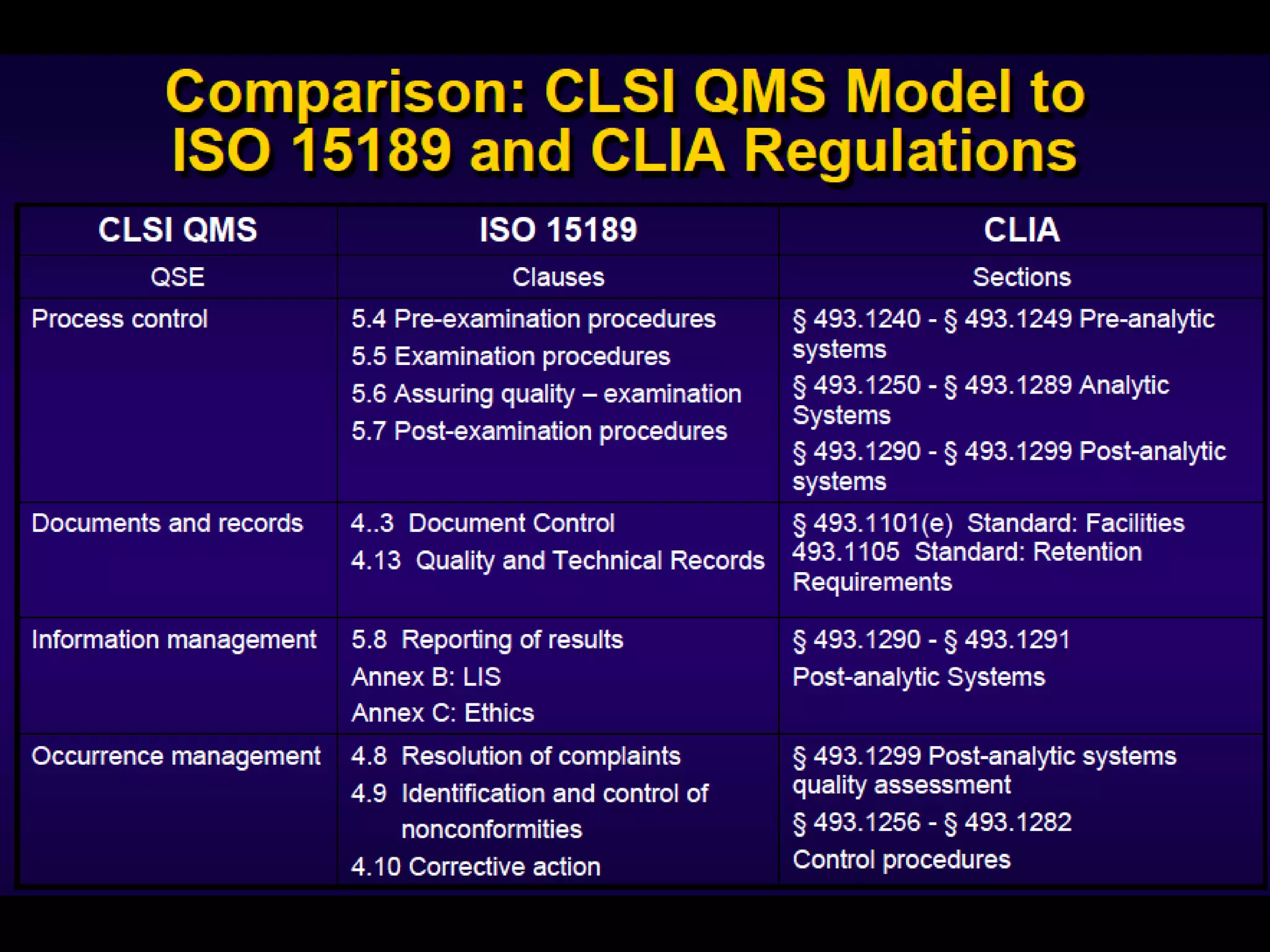
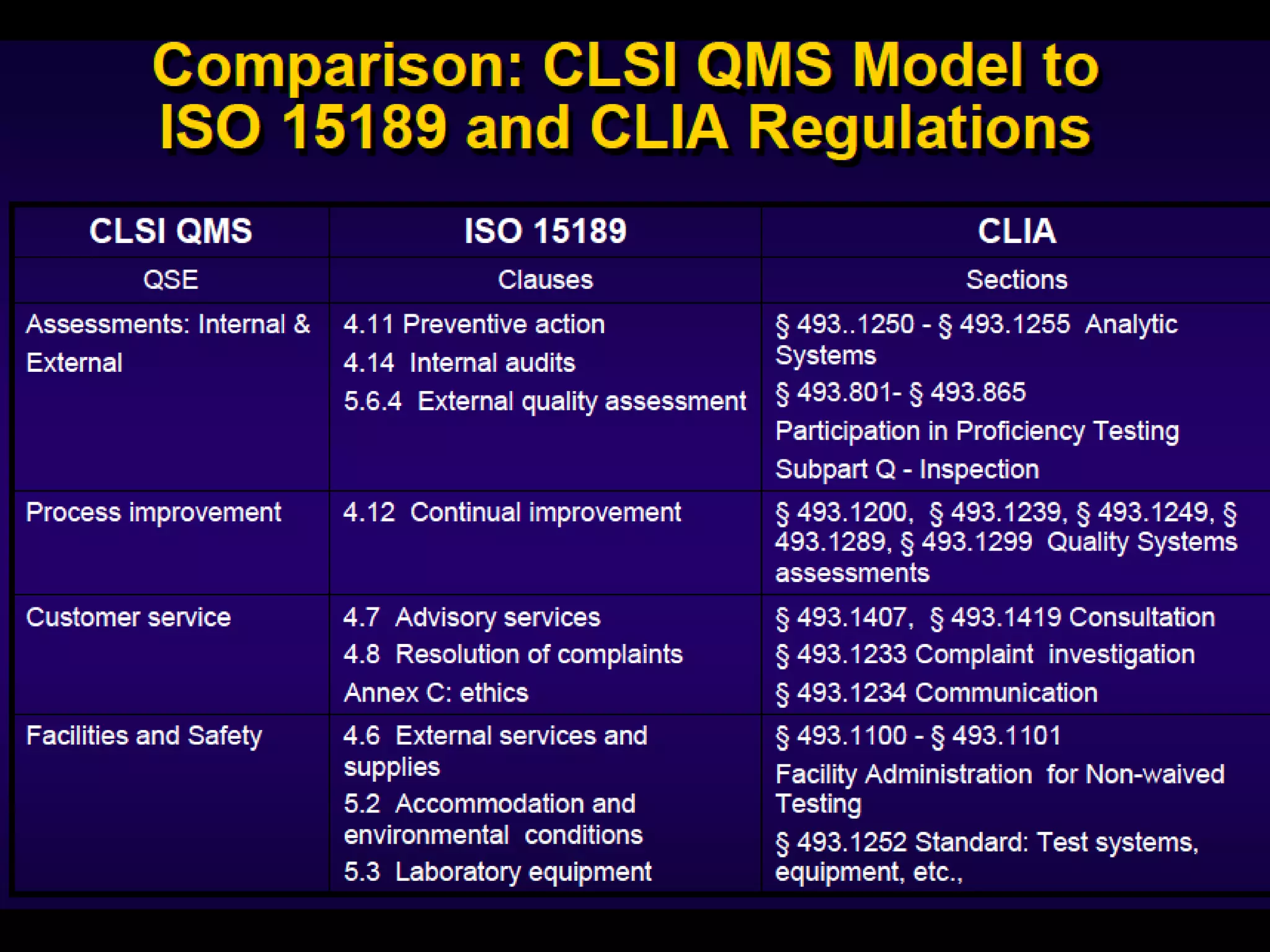
The document discusses regulatory requirements for clinical laboratories in the United States under the Clinical Laboratory Improvement Amendments (CLIA). It covers the federal agencies that oversee CLIA, how tests are categorized based on their complexity, quality control and quality assurance procedures required by CLIA, and the role and responsibilities of the laboratory director. The document also provides an overview of how to establish a CLIA-certified laboratory, including quality management systems, method validation, and other regulatory requirements.