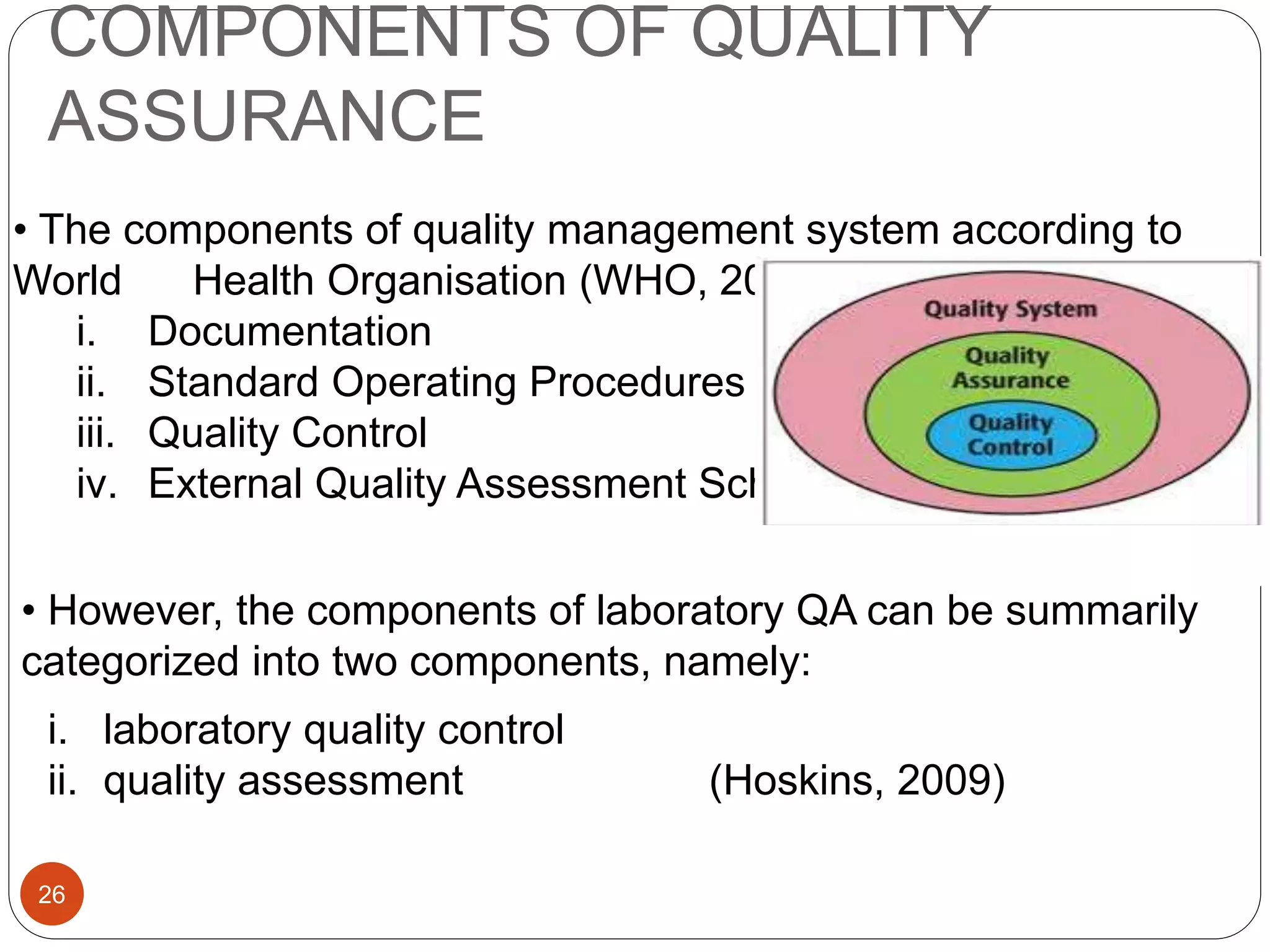
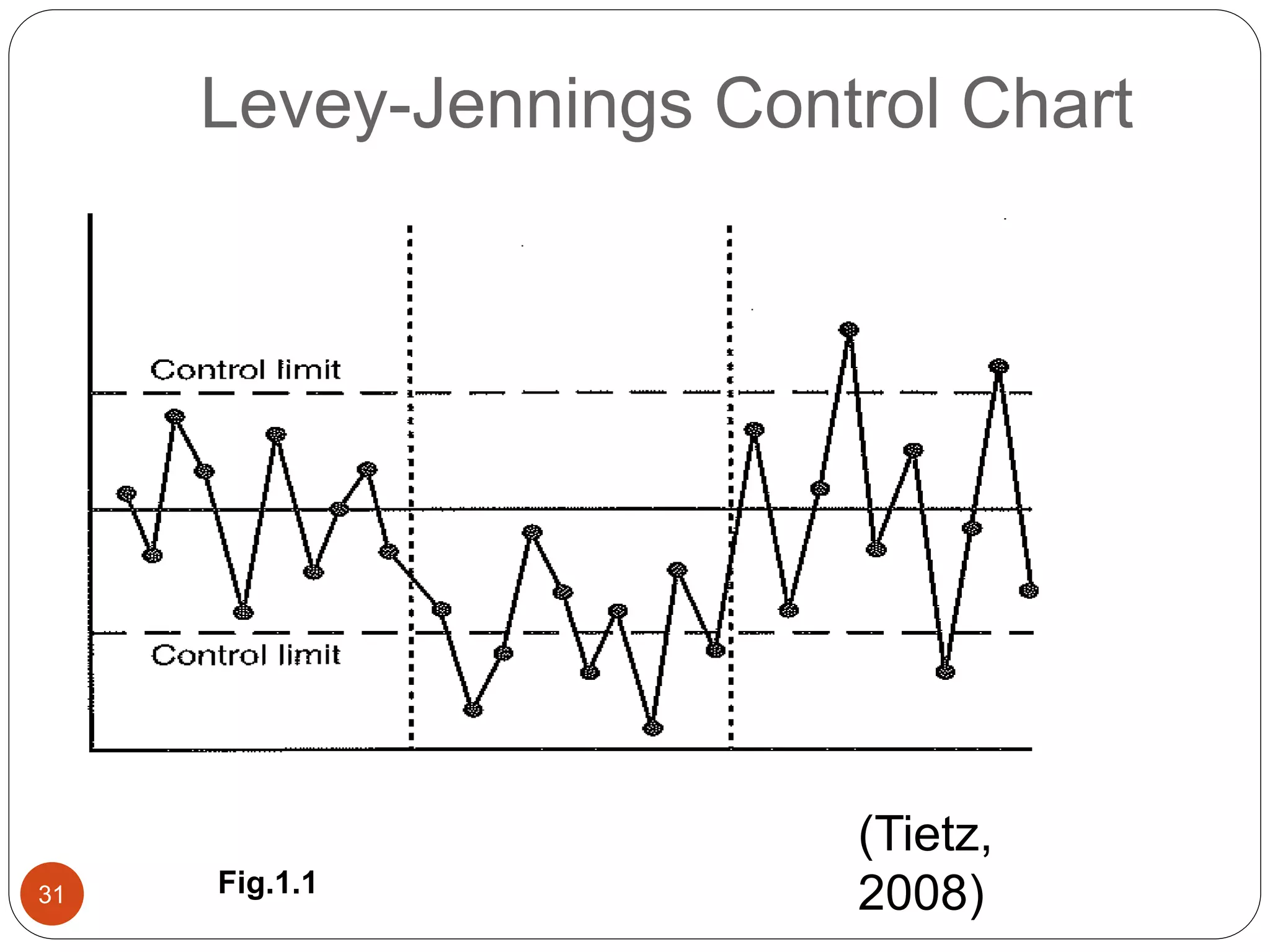
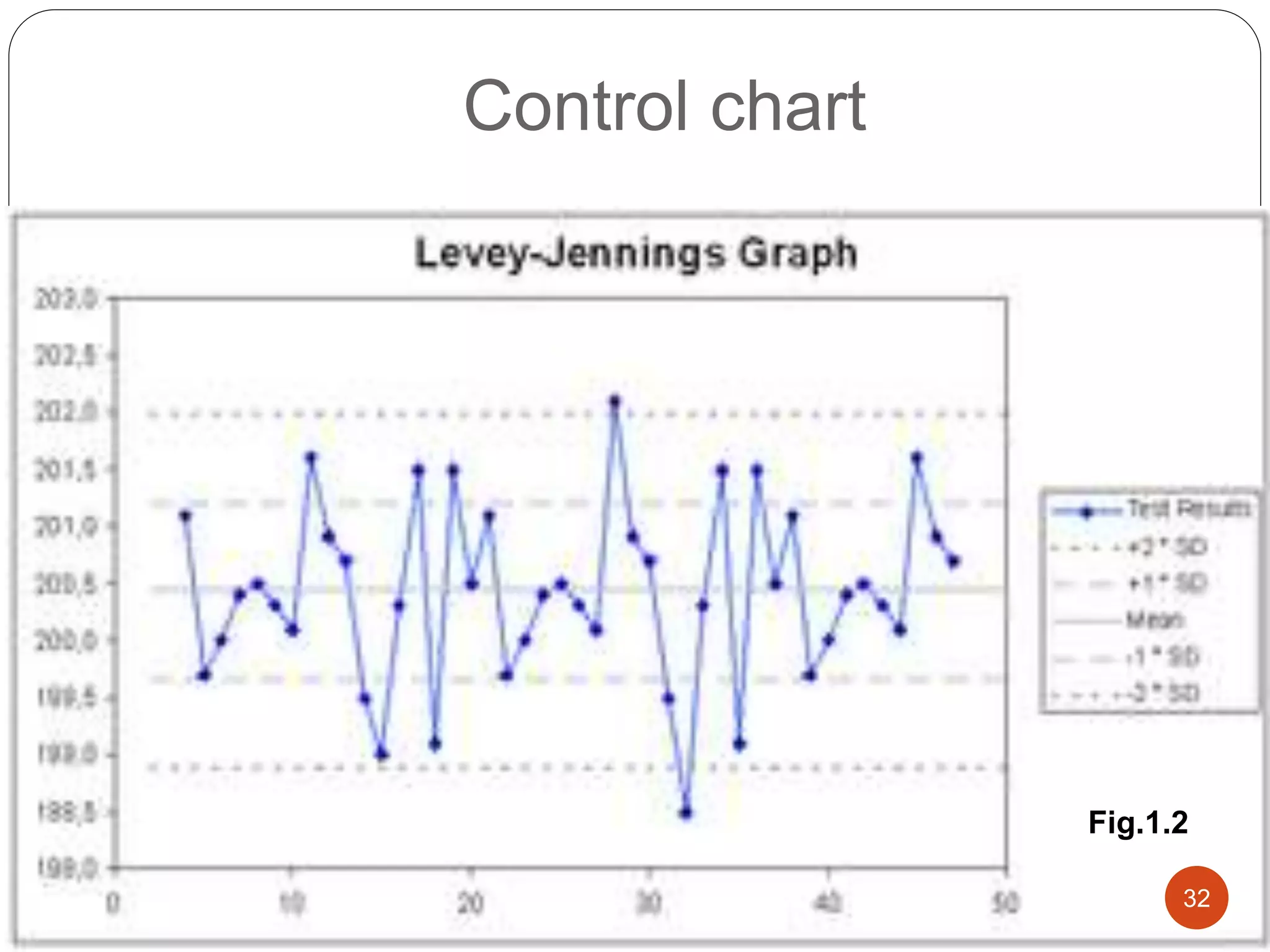
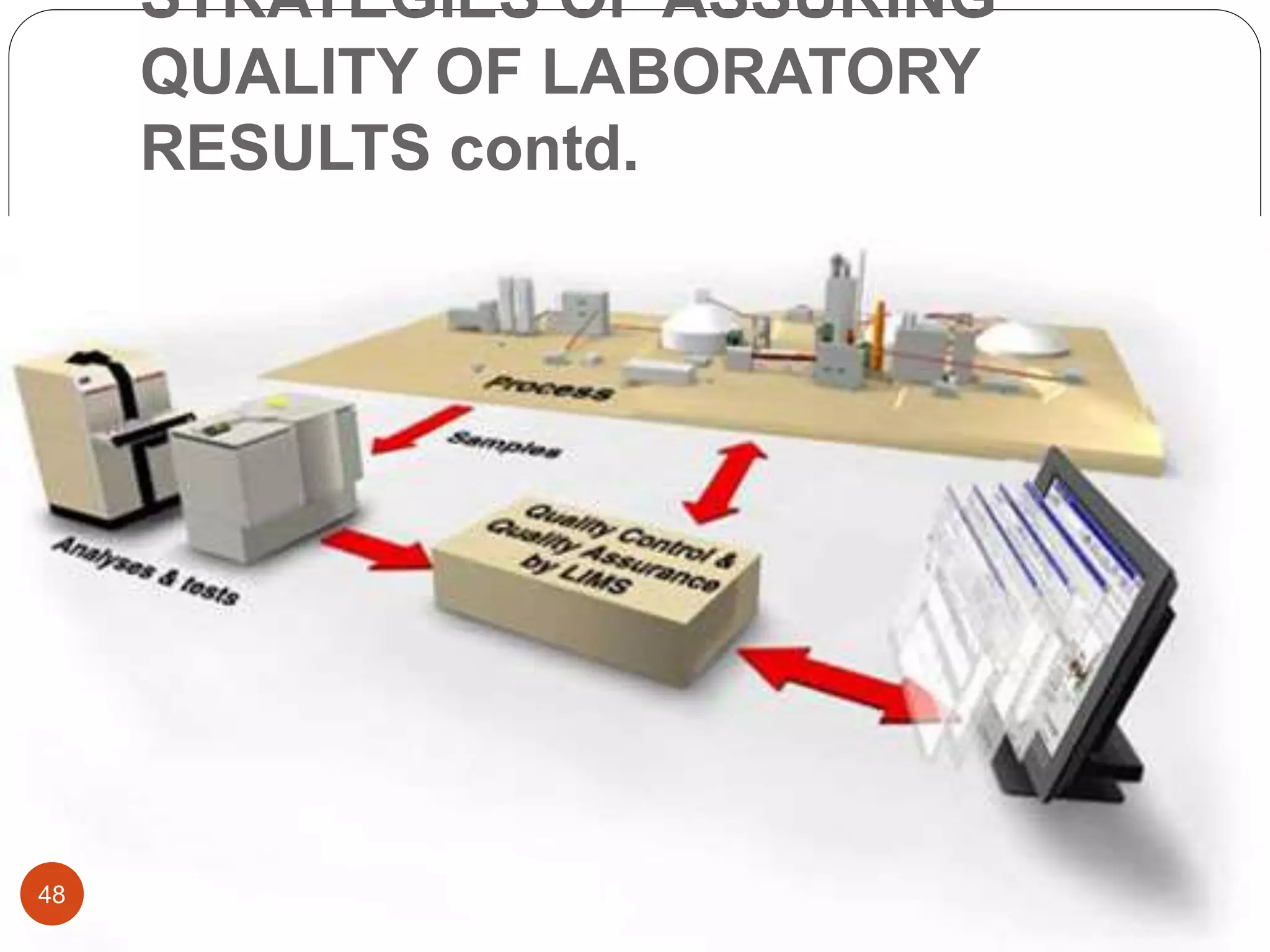
The document discusses strategies for assuring the quality of laboratory results. It covers quality assurance procedures such as quality control, quality assessment, laboratory standardization, and method validation. The key components of quality assurance include quality control, which uses techniques like analysis of reference materials and proficiency testing. Quality assessment involves periodic external evaluation to monitor and improve laboratory performance. Together, effective quality control and assessment help laboratories consistently produce accurate and reliable test results.