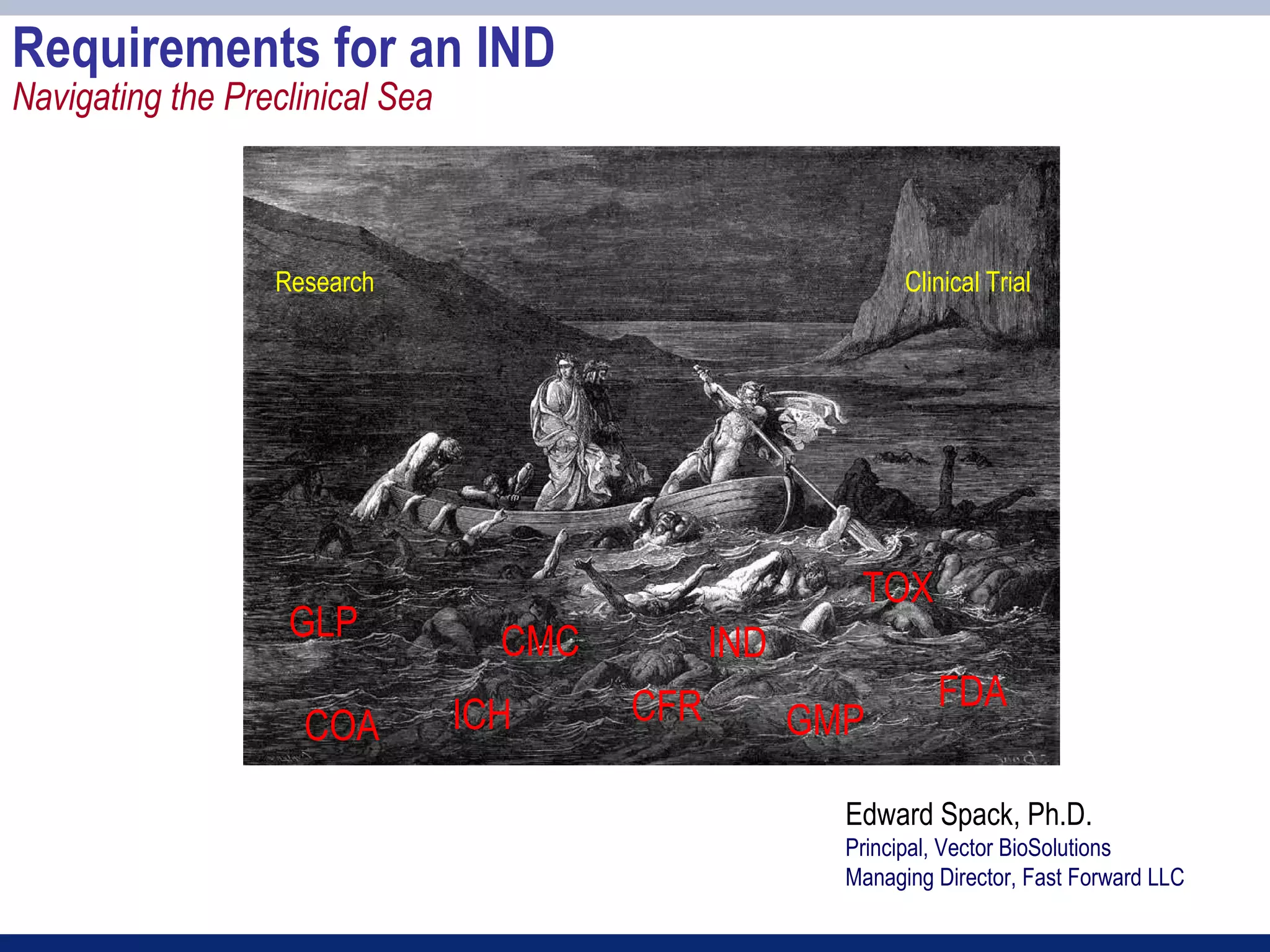
The document provides an overview of requirements for an Investigational New Drug (IND) application to the FDA. It discusses key components of the IND including chemistry, manufacturing and controls (CMC), preclinical toxicology studies, and clinical trial protocols. The main points are:
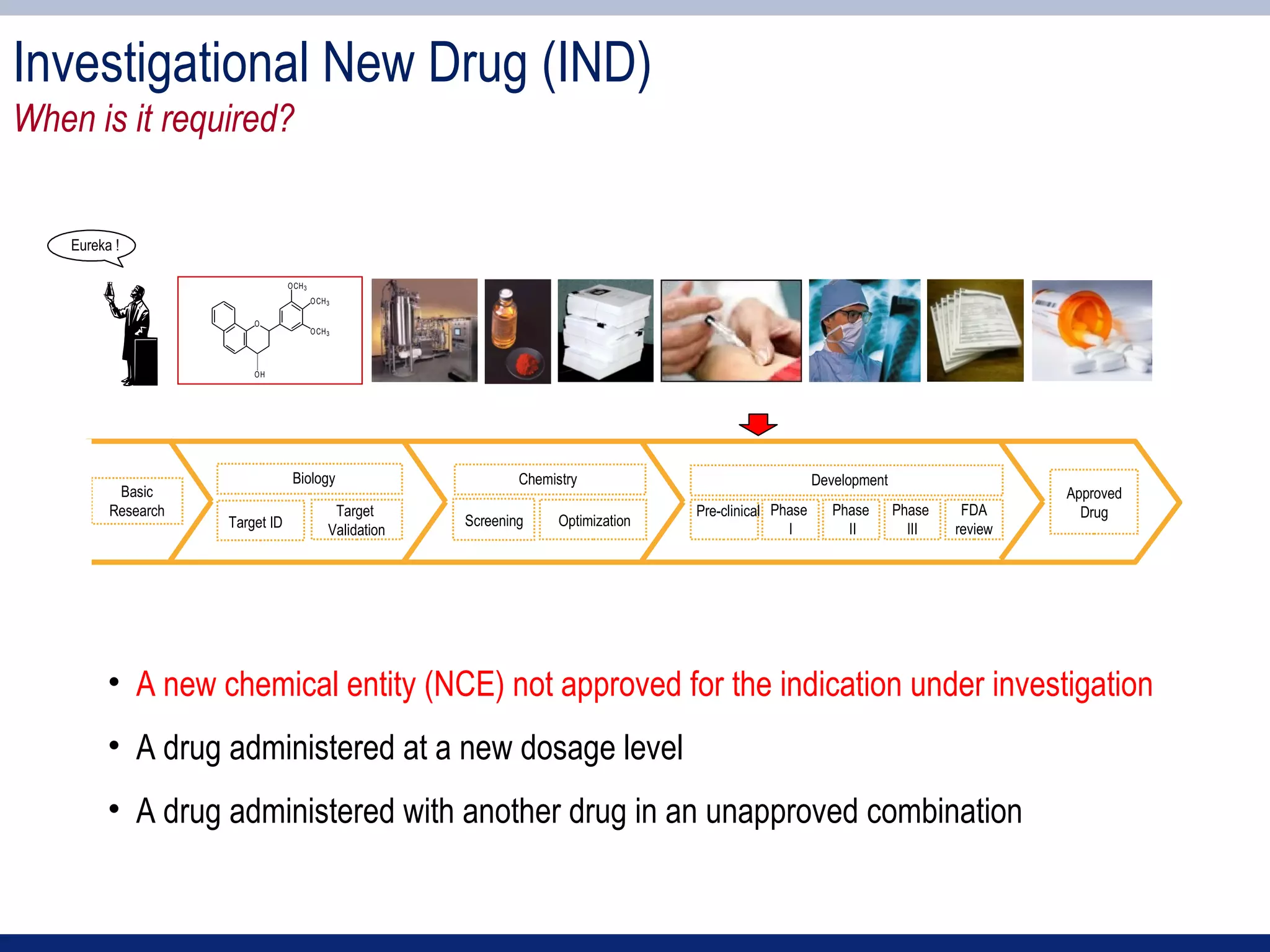
1) An IND application is required to begin clinical testing of new drugs, drugs at new dosages, or drug combinations not previously approved.
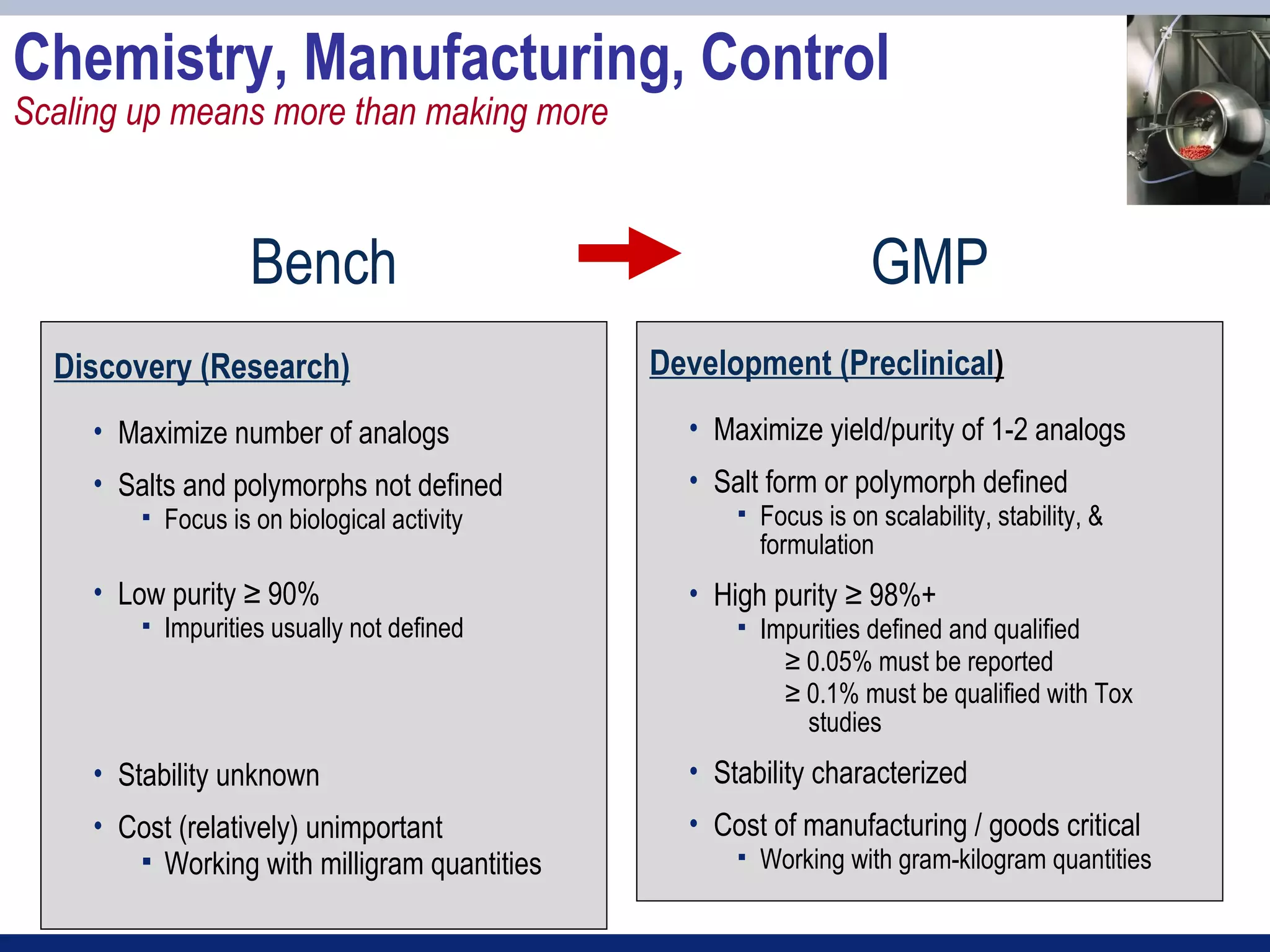
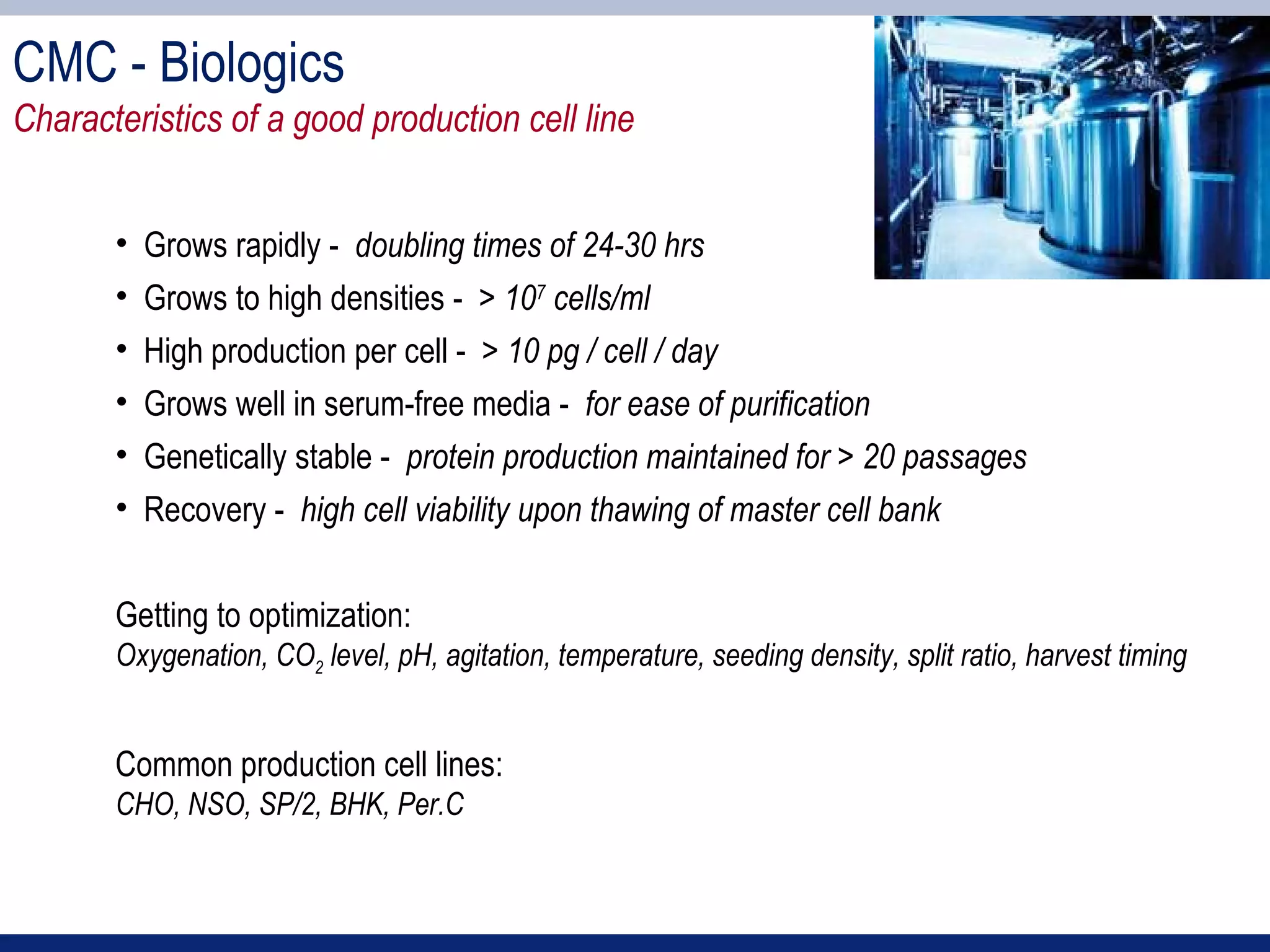
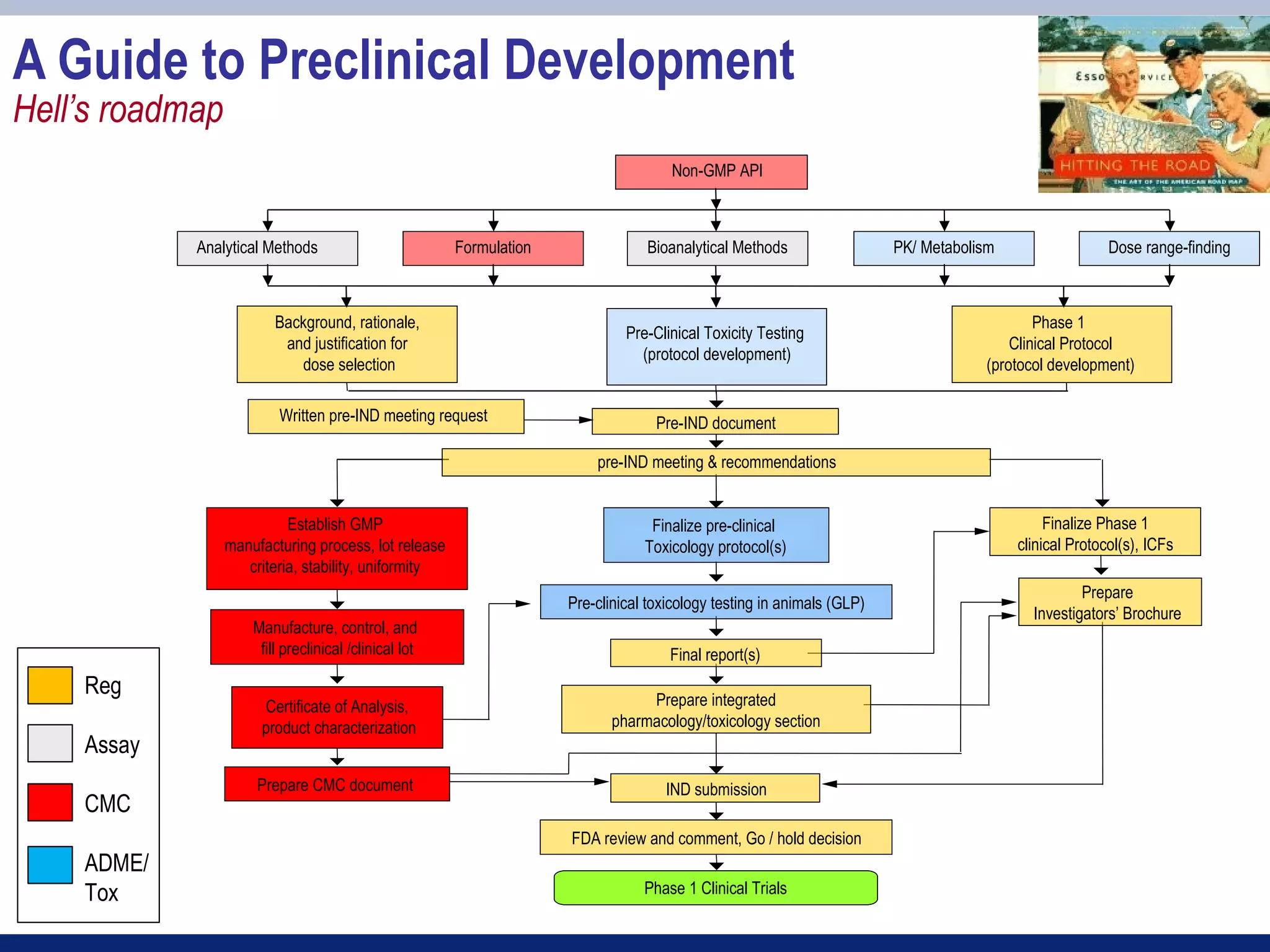
2) Key sections of the IND include CMC data on drug manufacturing and quality controls, results of preclinical toxicology studies in animals, and protocols for proposed clinical trials.
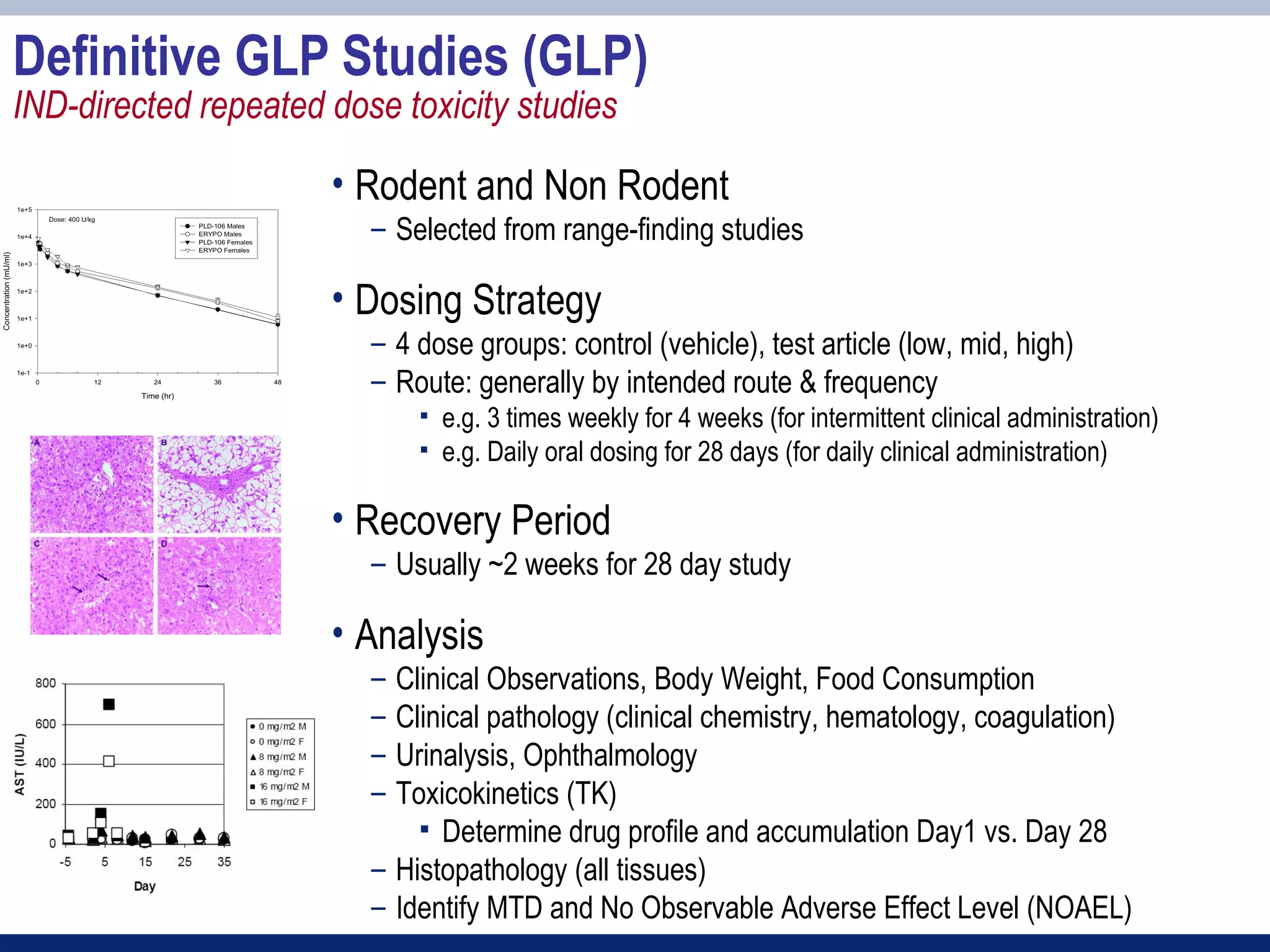
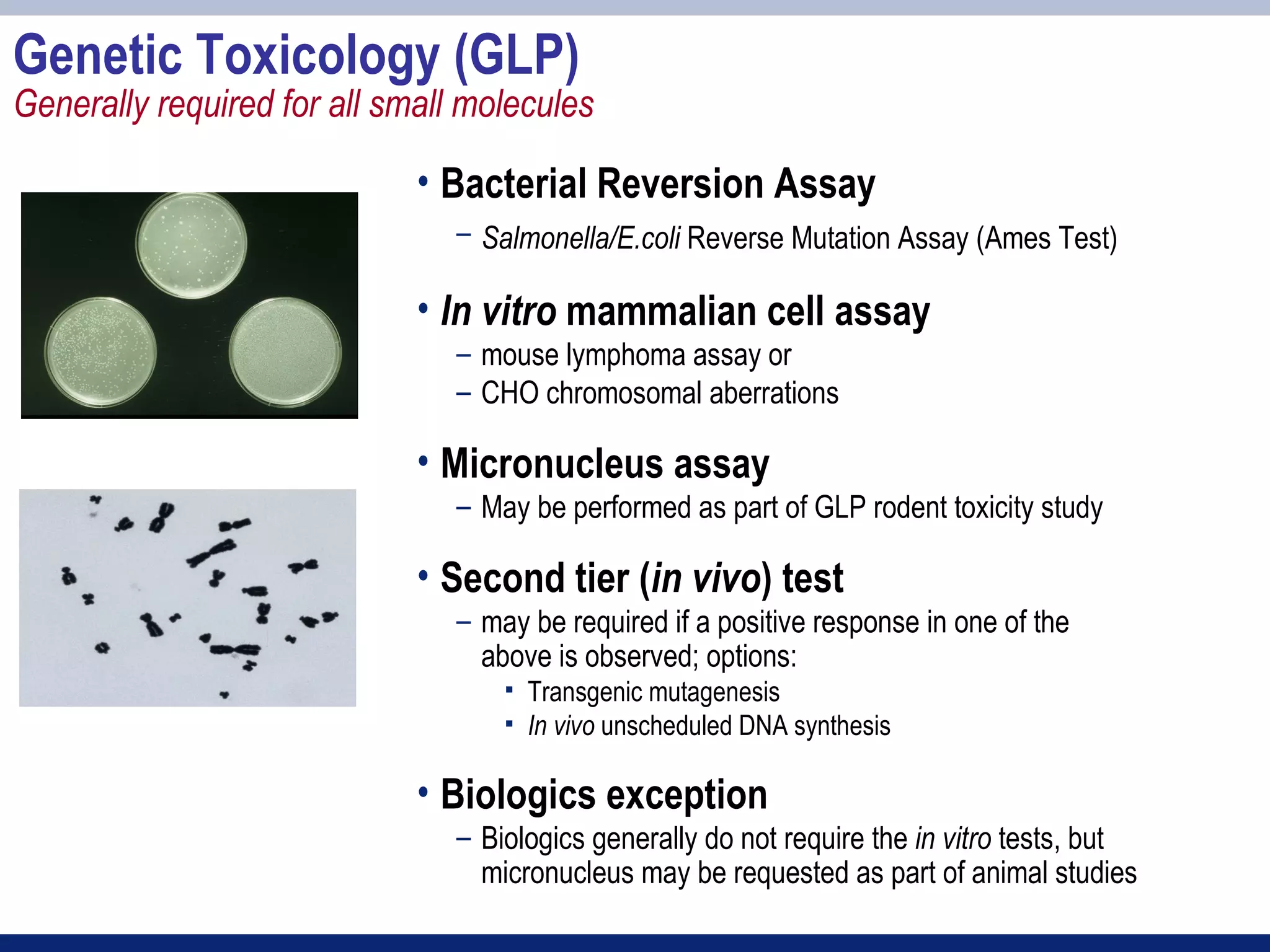
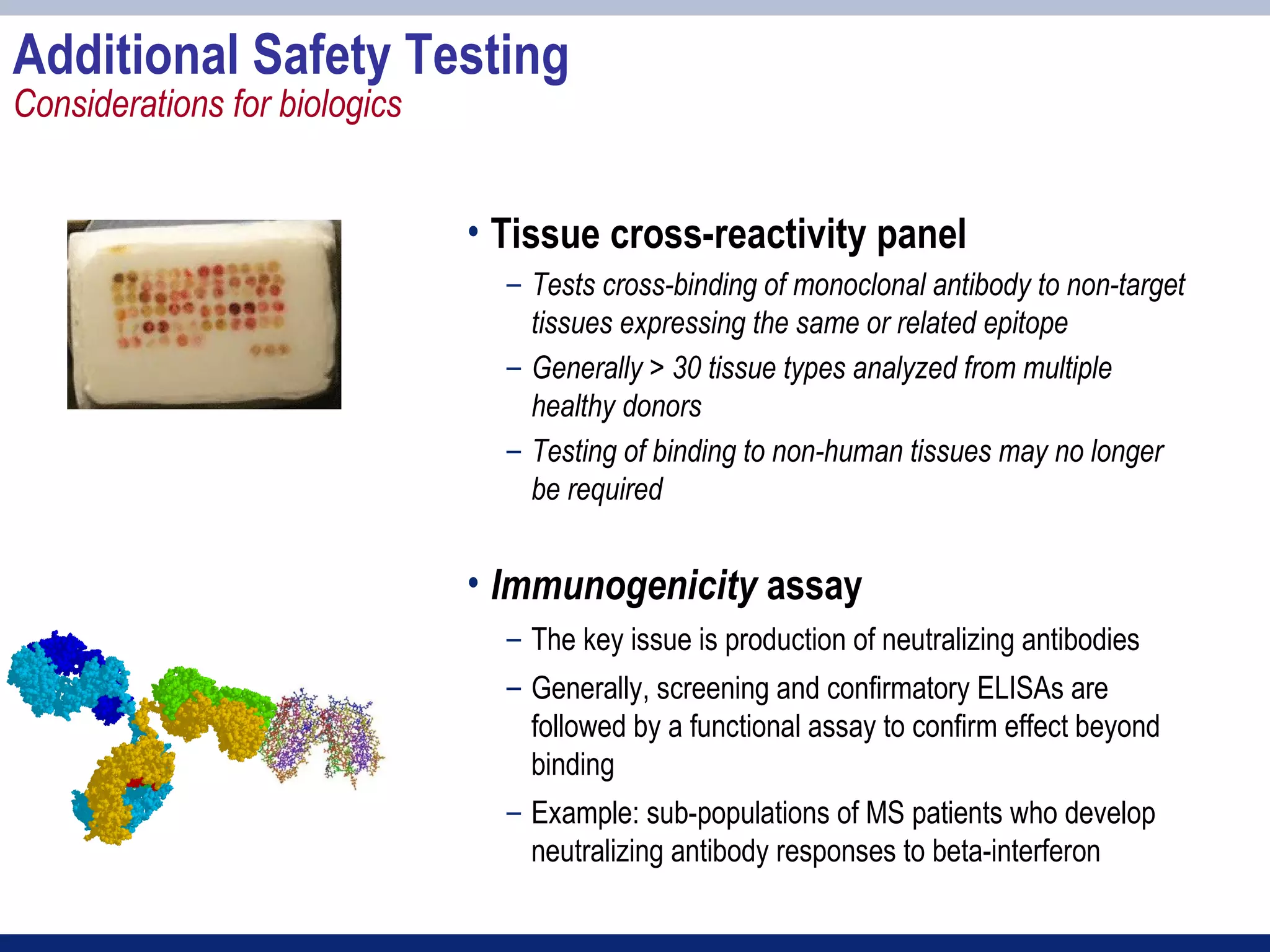
3) Preclinical studies aim to identify safe starting doses for clinical trials and target organs of toxicity.




![IND Components
IND Application Table of Contents (from FDA form 1571)
1. Form FDA 1571 [21 CFR 312.23(a)(1)]
2. Table of Contents [21 CFR 312.23(a)(2)]
3. Introductory statement [21 CFR 312.23(a)(3)]
4. General Investigational plan [21 CFR 312.23(a)(3)]
5. Investigator’s brochure [21 CFR 312.23(a)(5)]
6. Protocol(s) [21 CFR 312.23(a)(6)]
a. Study protocol(s) [21 CFR 312.23(a)(6)]
b. Investigator data [21 CFR 312.23(a)(6)(iii)(b)] or completed Form(s) FDA 1572
c. Facilities data [21 CFR 312.23(a)(6)(iii)(b)] or completed Form(s) FDA 1572
d. Institutional Review Board data [21 CFR 312.23(a)(6)(iii)(b)] or completed Form(s) FDA 1572
7. Chemistry, manufacturing, and control data [21 CFR 312.23(a)(7)]
Environmental assessment or claim for exclusion [21 CFR 312.23(a)(7)(iv)(e)]
8. Pharmacology and toxicology data [21 CFR 312.23(a)(8)]
9. Previous human experience [21 CFR 312.23(a)(9)]
10. Additional information [21 CFR 312.23(a)(10)]](https://image.slidesharecdn.com/session3part2-120309153702-phpapp02/75/Session-3-part-2-7-2048.jpg)










![Acknowledgements
It takes a team
CMC
• Alex Kostrikin [Anergen – PharmaCyclics]
• Milan Tomic [Xoma]
Toxicology
• Nancy Wehner [Anergen – Consultant]
• Karen Steinmetz [SRI International]
• Jon Mirsalis [SRI International]
Project Management
• Tom Yonker [InterMune – KaloBios]
• Joanne Snow [Snow Associates]](https://image.slidesharecdn.com/session3part2-120309153702-phpapp02/75/Session-3-part-2-29-2048.jpg)