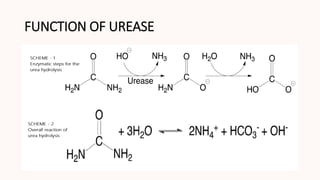
The document provides an overview of the mechanisms of enzyme action for trypsin, chymotrypsin, and urease. It discusses the structure and catalytic function of these enzymes, detailing the catalytic triad for serine proteases and the role of urease in the hydrolysis of urea. It also highlights ongoing research questions surrounding urease's activation process and its accessory proteins.