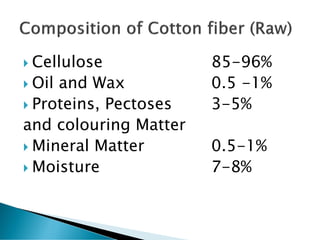
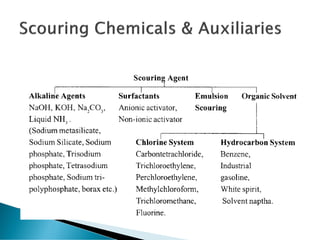
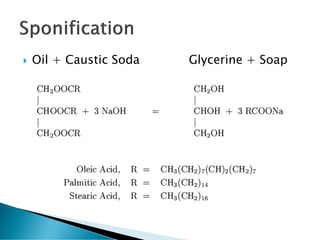
The document discusses the scouring process used to clean fabrics prior to dyeing or finishing. Scouring removes natural and mechanical impurities through processes like saponification, solubilization, and emulsification. Saponification converts oils and fats to soap and glycerin. Solubilization and emulsification remove other impurities like pectins, proteins, waxes, and minerals by making them water soluble or dispersing them. Selection of scouring agents depends on fiber type, fabric properties, and impurity level. Effectiveness is tested through measures like absorbency, weight loss, and residual wax content.