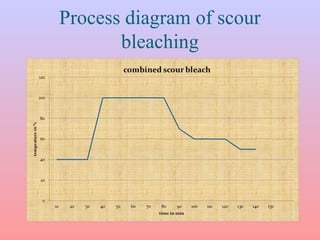
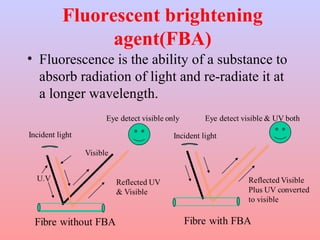
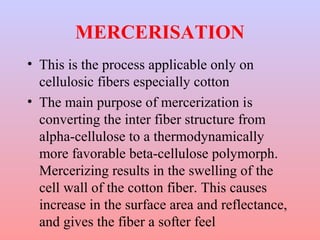
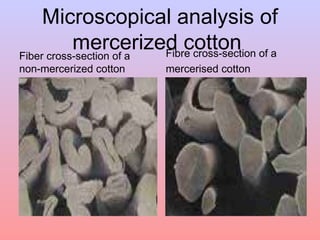
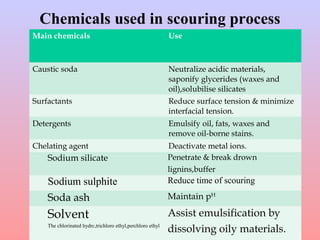
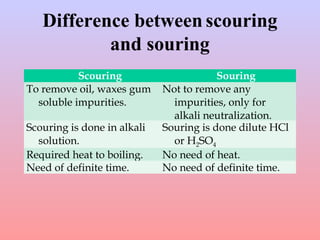
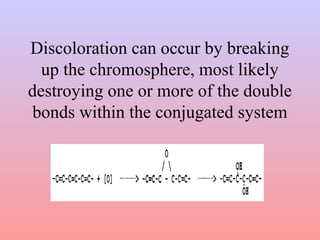
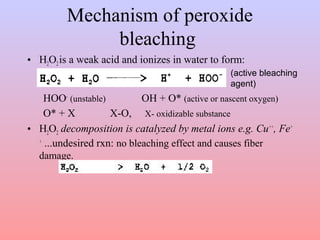
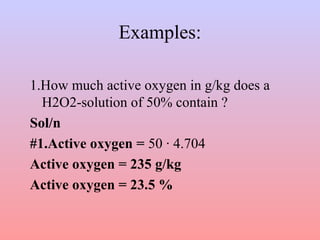The document discusses chemical processing of knitted fabrics, specifically preparatory processes like scouring and bleaching. It describes the objectives of preparatory processes as removing natural and added impurities to allow for high dye uptake and brightness. Scouring removes oils, waxes and other impurities using alkalis and surfactants while bleaching destroys coloring matter using oxidizing agents like hydrogen peroxide and sodium hypochlorite. Key steps and considerations for each process are outlined, including chemicals used, mechanisms, effects of parameters, and common tests after pretreatment.












































![• Out of 34.0146g H2O2 100 % result from
½ · 31.9988g =15.9994g of so-called
active oxygen.
• The conversion factor of % by weight of
H2O2-solution in active oxygen is the
following:
active oxygen [g/kg] = % in weight H2O2
solution *4.704](https://image.slidesharecdn.com/cottonpreparationppt-150106002541-conversion-gate01/85/Cotton-preparation-ppt-50-320.jpg)