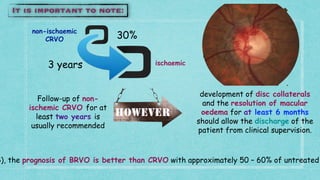
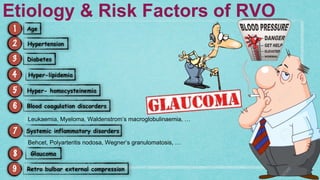
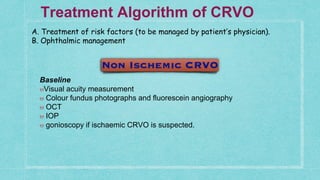
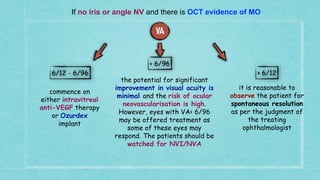
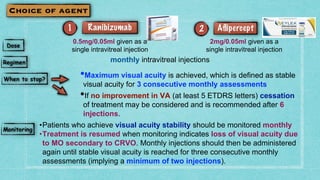
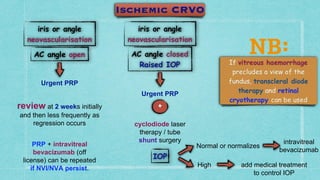
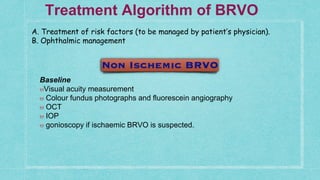
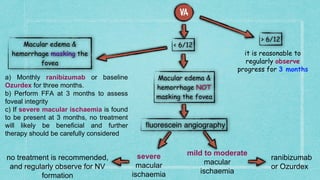
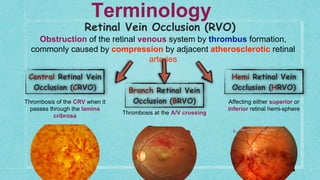
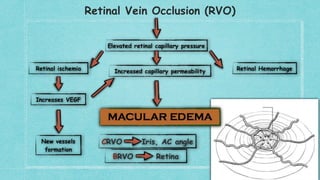
This document provides guidelines for the treatment of retinal vein occlusion (RVO). It defines RVO and distinguishes between central retinal vein occlusion (CRVO) and branch retinal vein occlusion (BRVO). For non-ischemic CRVO, the guidelines recommend regular observation or treatment with anti-VEGF drugs or steroid implants depending on the presence of macular edema. For ischemic CRVO or if neovascularization develops, prompt laser photocoagulation or intravitreal anti-VEGF therapy is recommended. The treatment guidelines for BRVO similarly involve regular observation, anti-VEGF drugs, or laser depending on the severity of macular ischemia or edema. Experimental treatments are also discussed but not generally recommended outside of clinical trials

![Ischaemic CRVO is associated with one or more of the following characteristics:
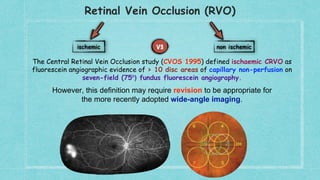
6. FA showing > 10 disc areas of retinal capillary non-perfusion on 7-field fluorescein angiography
7. ERG: reduced b wave amplitude, reduced b:a ratio and prolonged b-wave implicit time
Poor VA (44% of
eyes with vision of
<6/60 develop
rubeosis [CVOS]
RAPD
•Presence of multiple dark deep intra-
retinal haemorrhages
•Presence of multiple CWS
•Degree of retinal vein dilatation and
tortuosity](https://image.slidesharecdn.com/rvoguidelines-180401224448/85/Rvo-guidelines-5-320.jpg)