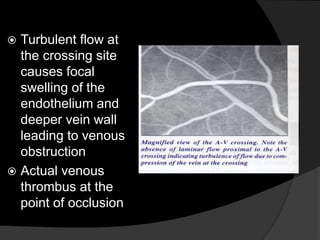
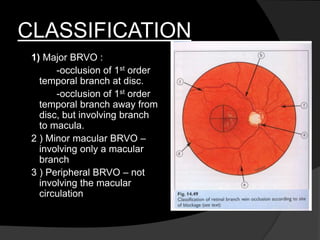
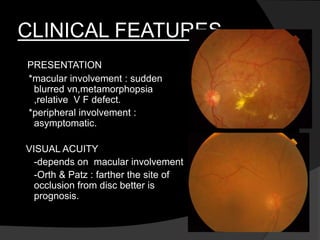
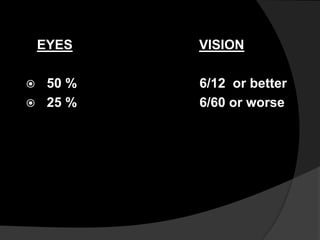
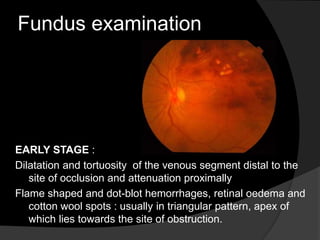
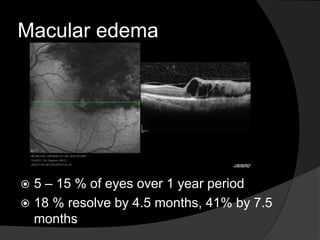
This document discusses branch retinal vein occlusion (BRVO), including its pathogenesis, risk factors, clinical features, investigations, and management approaches. Some key points:
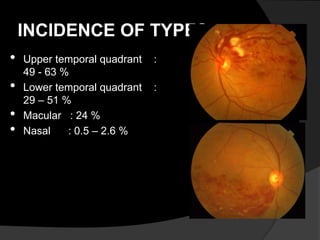
- BRVO is the second most common cause of vision loss due to retinal vascular disease, after diabetic retinopathy. It occurs most often in patients in their 50s-60s and is caused by obstruction of a branch retinal vein.
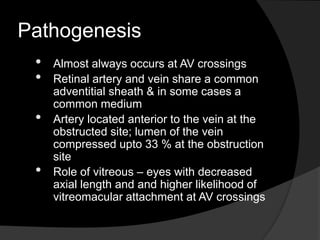
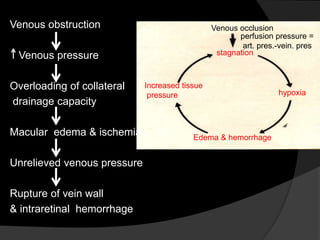
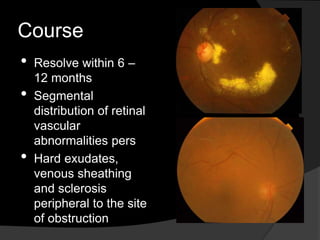
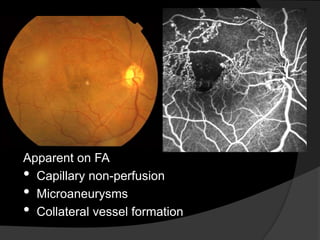
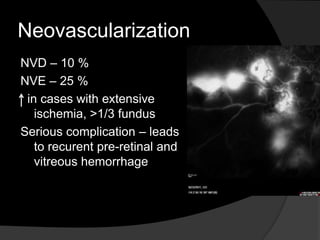
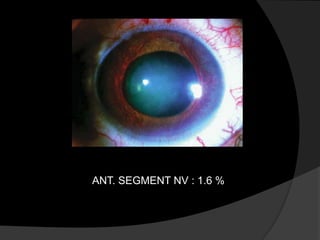
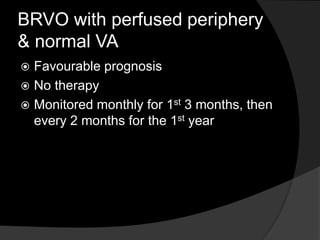
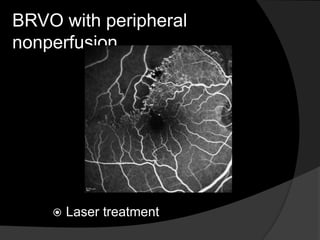
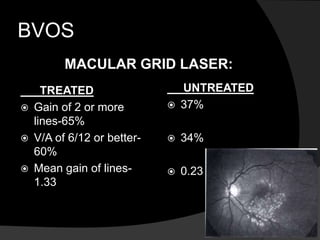
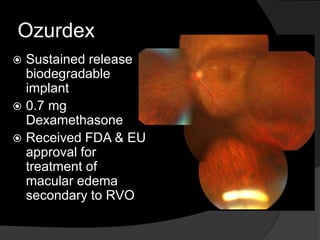
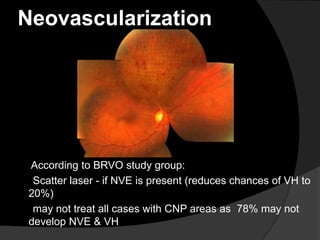
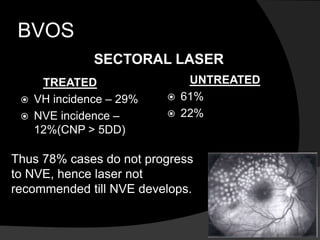
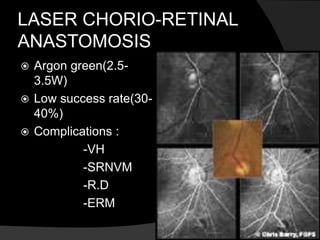
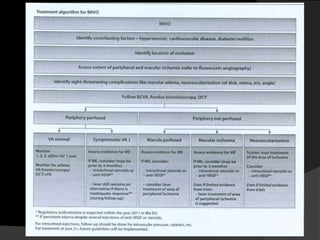
- Risk factors include hypertension, diabetes, hyperlipidemia, glaucoma, smoking, and age-related atherosclerosis. Laser treatment can help manage macular edema and neovascularization complications.
- Treatment aims to manage modifiable risk factors and sight-threatening complications like macular edema, non





















![Plasminogen
Activator to Treat Macular
Edema
Associated With Branch
Retinal
Vein Occlusion
(Am J Ophthalmol 2006;142: 318–320
• After topical anesthesia was applied and a
paracentesis created
• tPA (40 k international units [IU]) injected into the
vitreous
• bedrest in the supine position for four hours
following treatment
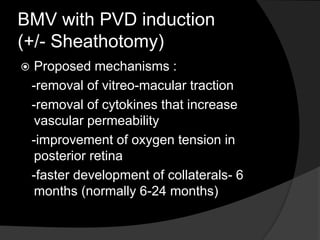
Mechanisms - PVD induction & development of
collateral vessels](https://image.slidesharecdn.com/brvo-170726100402/85/Branch-Retinal-Vein-Occlusion-62-320.jpg)



