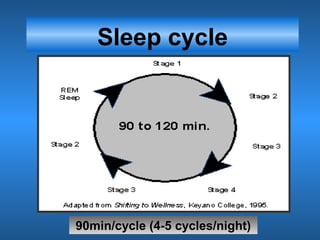
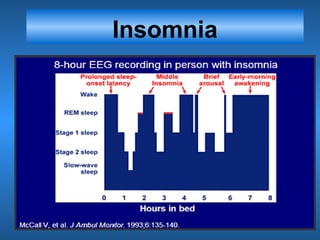
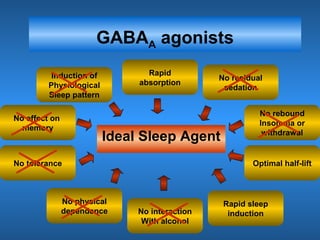
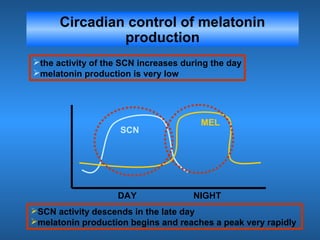
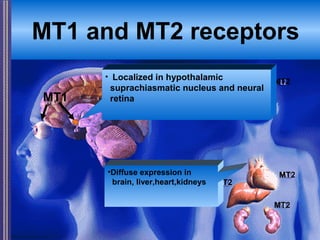
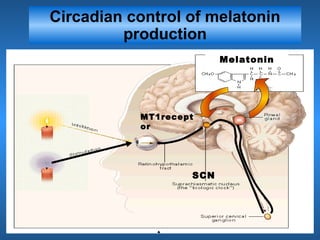
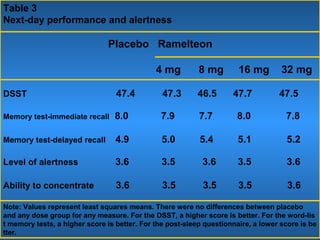
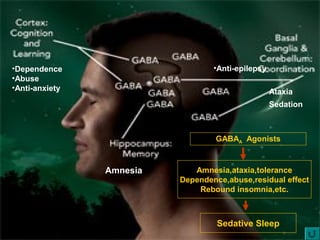
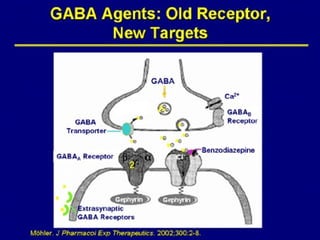
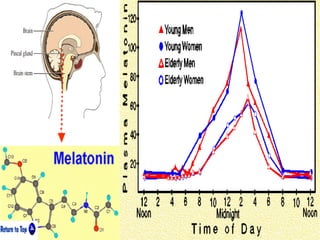
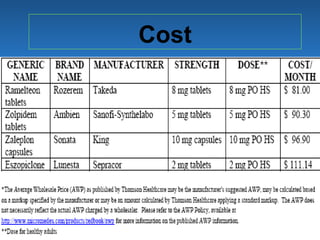
This document discusses the melatonin receptor agonist ramelteon, which is approved for the treatment of insomnia. It summarizes ramelteon's mechanism of action as a highly selective agonist for melatonin receptors MT1 and MT2, which are involved in regulating sleep-wake cycles. Clinical studies showed that ramelteon significantly reduced time to fall asleep and increased total sleep time compared to placebo, without next-day residual effects. In contrast, benzodiazepines and other sedative-hypnotics can cause dependence, abuse potential, and daytime sedation. Ramelteon has no serious adverse effects and no abuse potential even at high doses, making it preferable to other