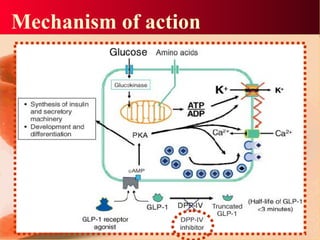
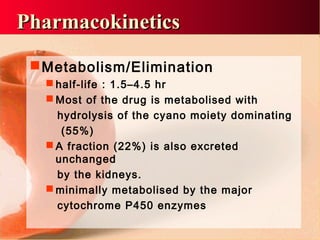
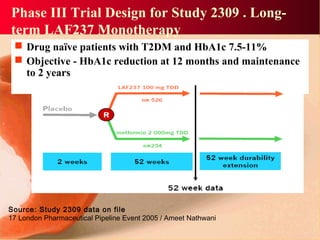
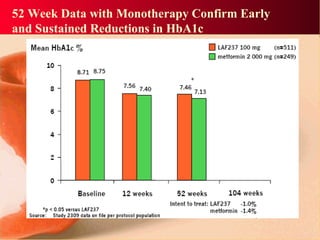
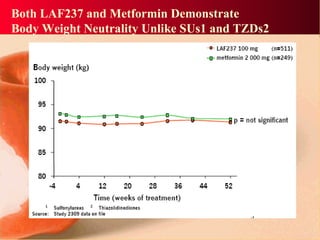
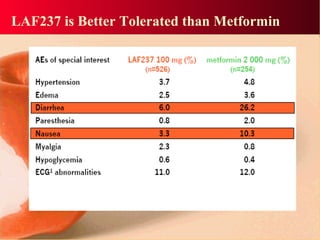
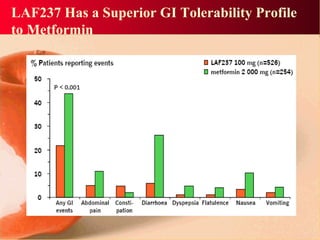
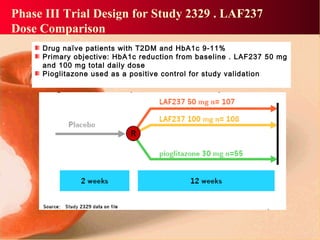
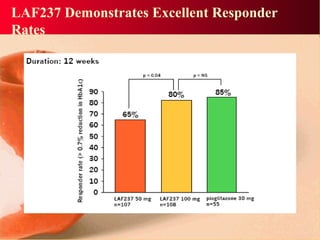
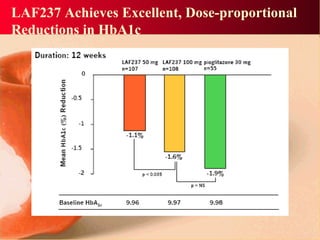
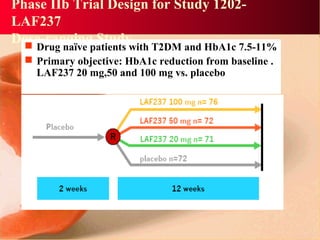
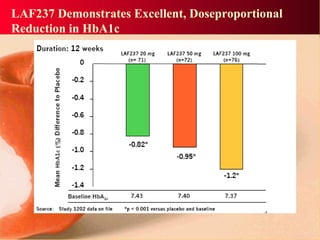
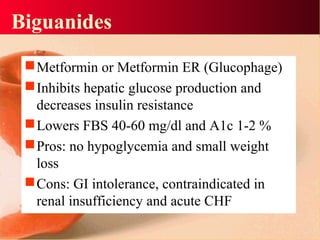
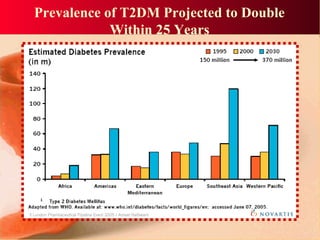
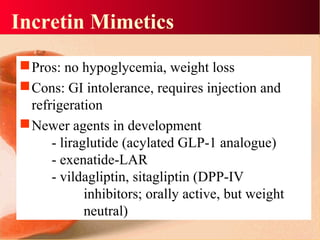
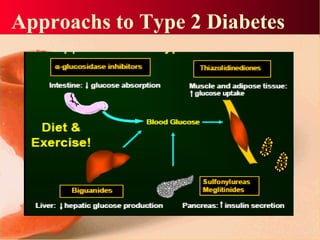
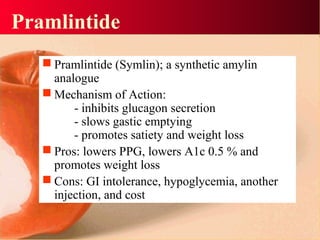
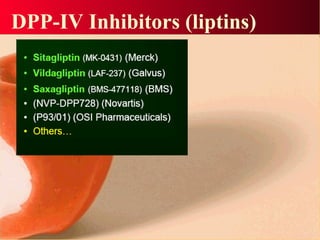
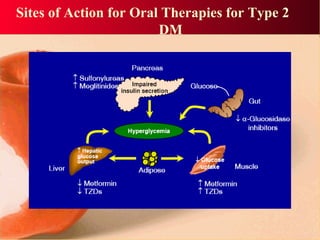
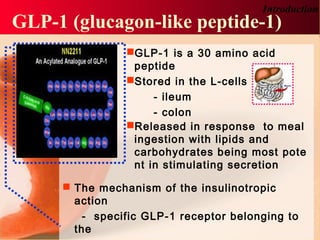
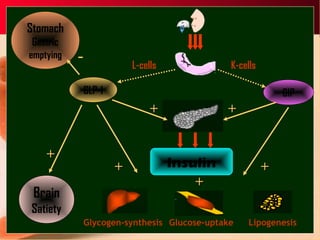
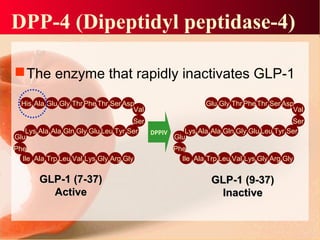
Vildagliptin is a DPP-IV inhibitor used to treat type 2 diabetes. It works by inhibiting the DPP-IV enzyme, which helps increase levels of incretin hormones like GLP-1. This enhances the effects of GLP-1, including stimulating more insulin release from beta cells and suppressing glucagon secretion. Clinical studies showed vildagliptin lowers blood glucose levels and is well tolerated with a low risk of hypoglycemia and no weight gain.










![Vildagliptin
•the first in a new class of oral antidiabetic agents
•known as dipeptidyl peptidase IV inhibitors
(DPP-IV) inhibitors
"incretin enhancers"
(2S)-([(3-hydroxyadamantan-1-yl)
amino]acetyl)-pyrrolidine-2-carbonitrile](https://image.slidesharecdn.com/vildagliptin-150308075831-conversion-gate01/85/Vildagliptin-14-320.jpg)