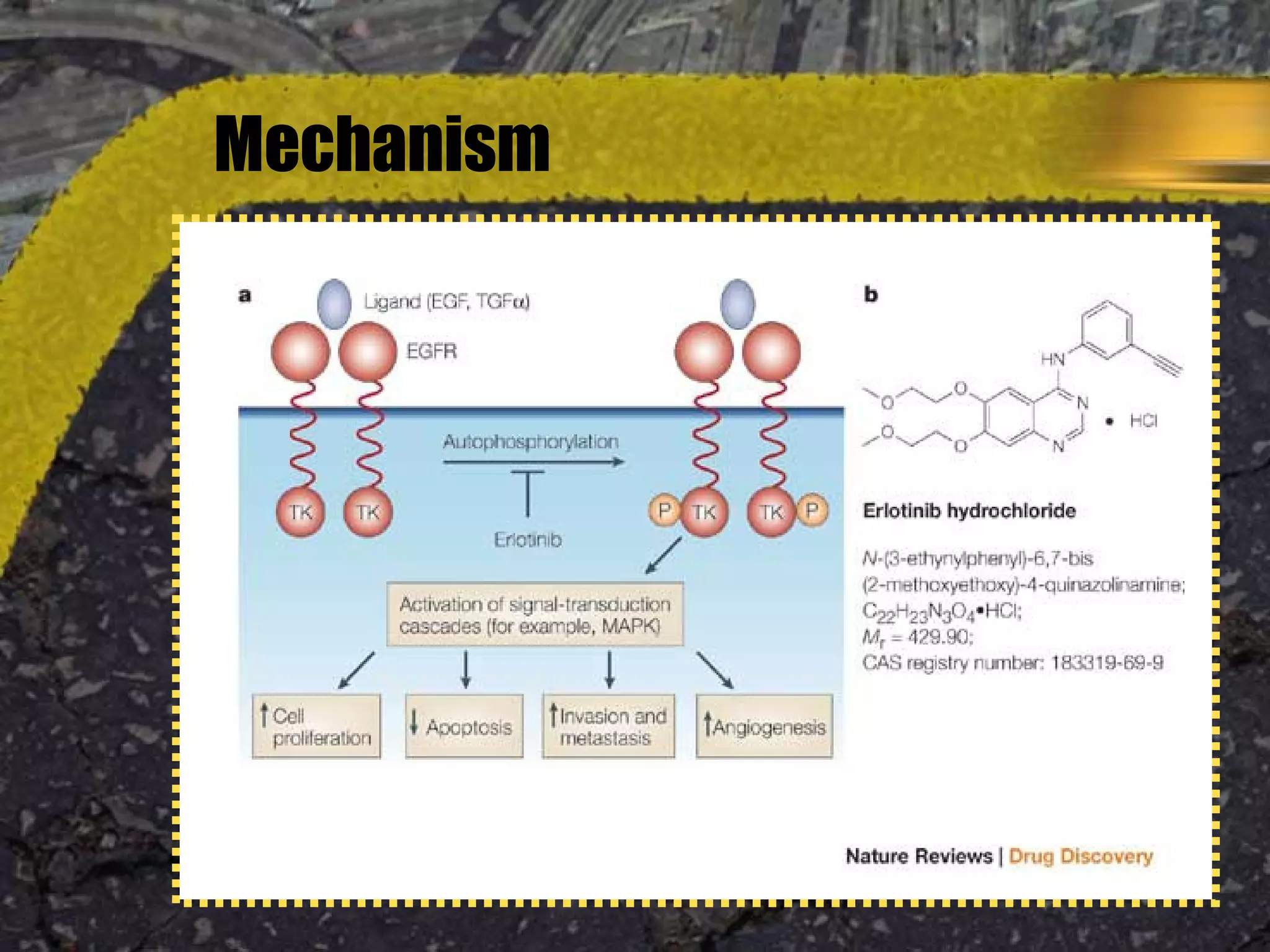
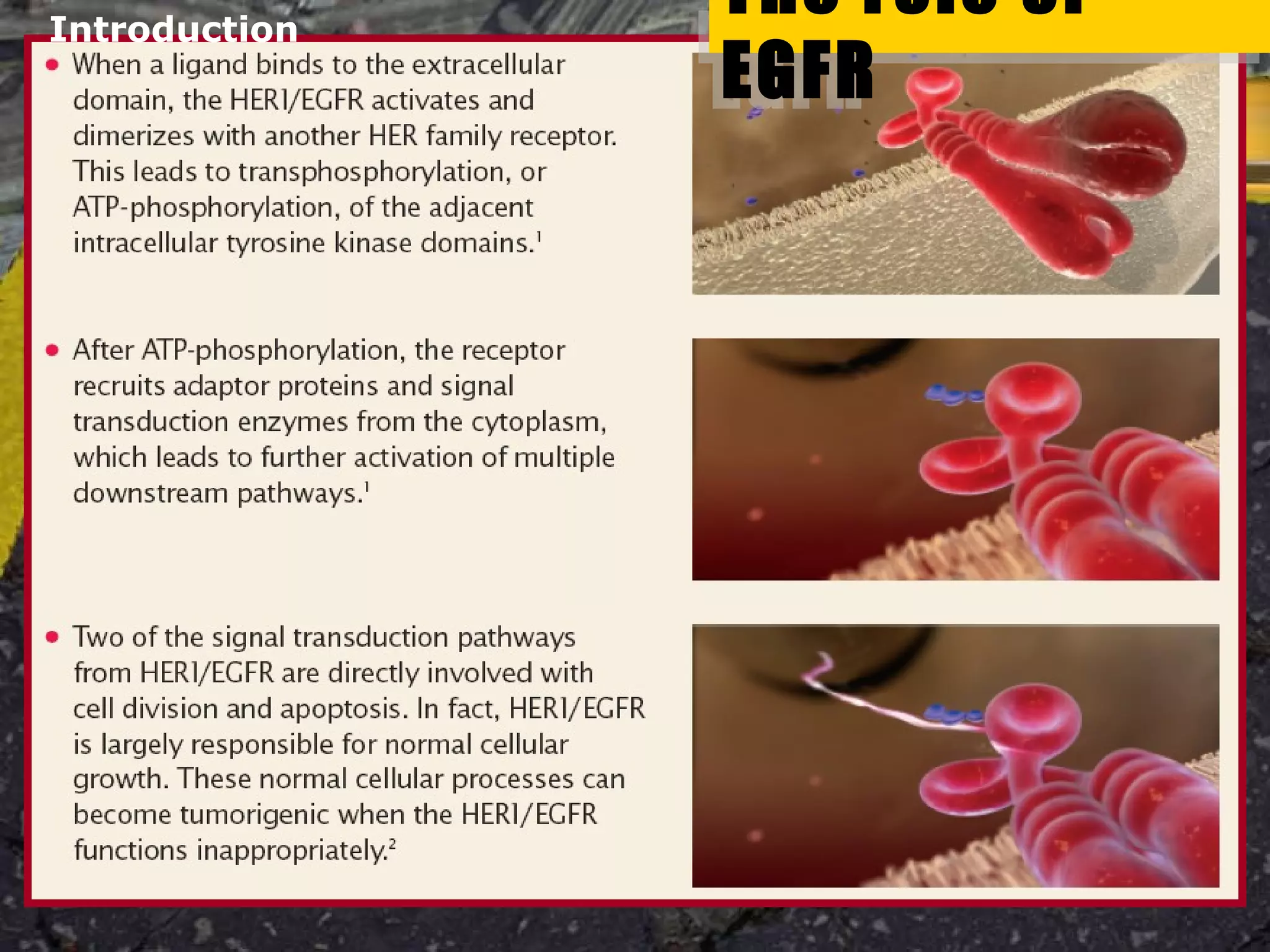
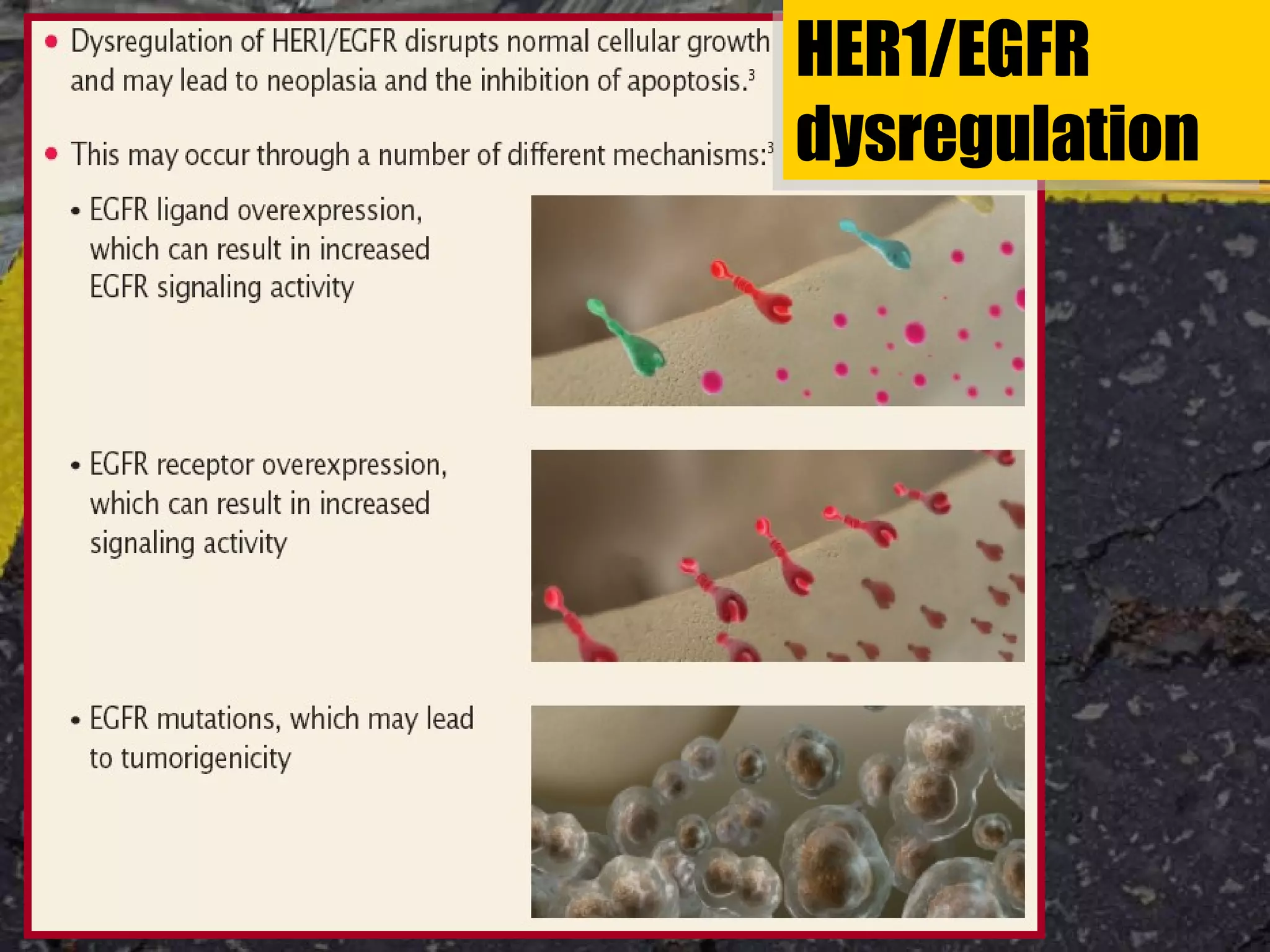
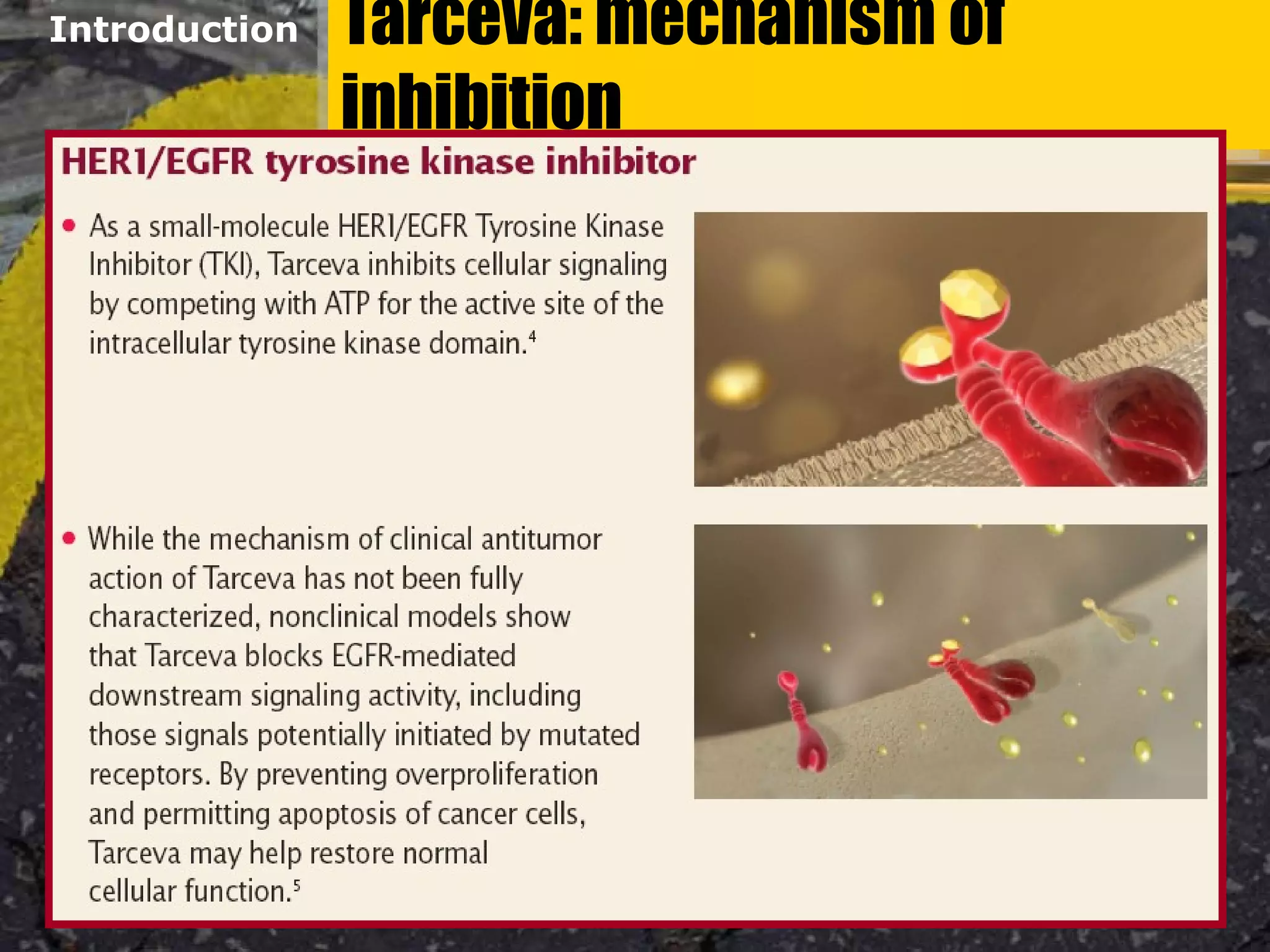
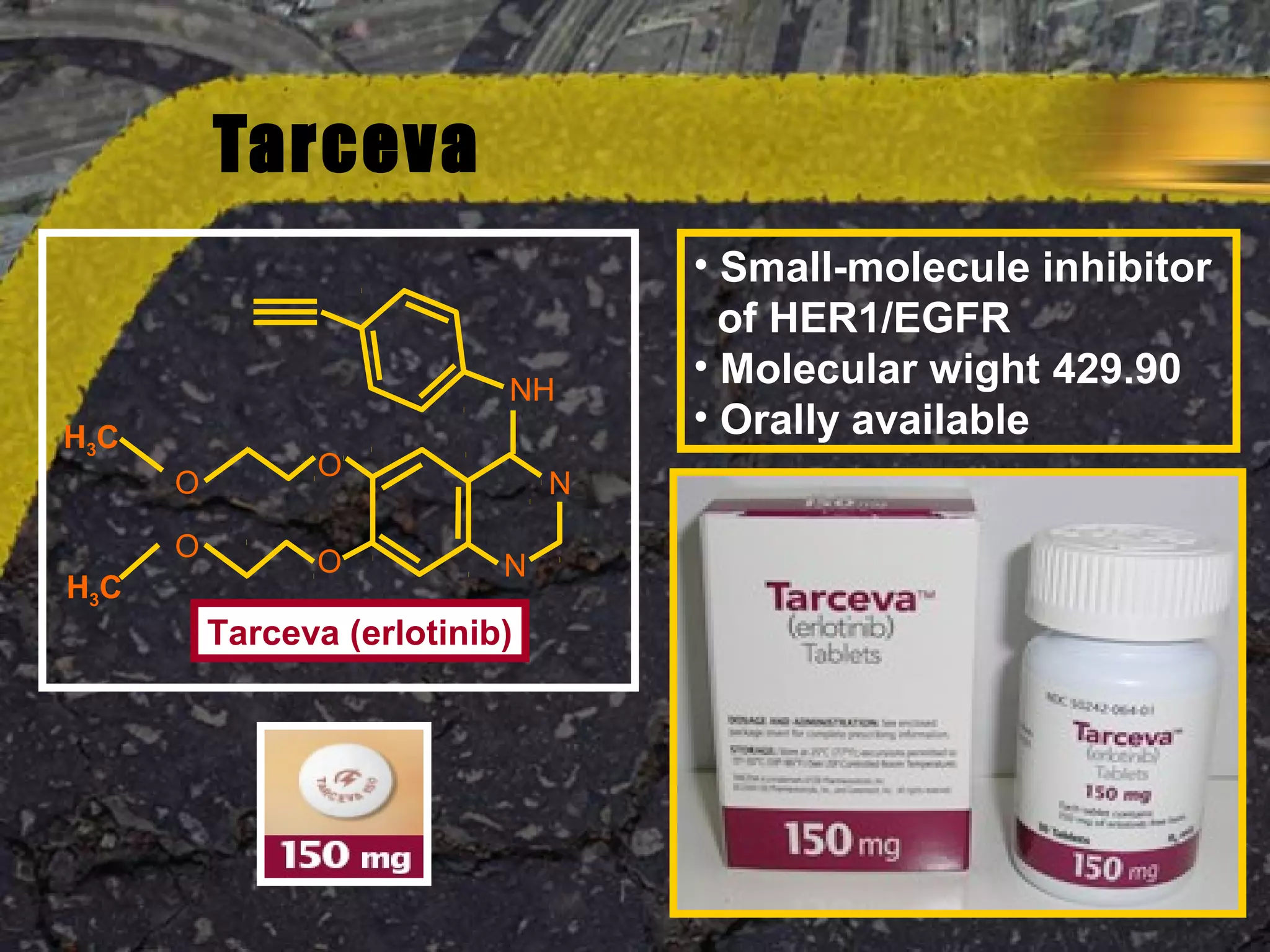
Tarceva (erlotinib) is an oral targeted therapy approved for treating locally advanced or metastatic non-small cell lung cancer (NSCLC) that has progressed after chemotherapy. It works by inhibiting the HER1/EGFR tyrosine kinase receptor, blocking cancer cell proliferation. Clinical trials showed Tarceva improved progression-free and overall survival compared to placebo in NSCLC patients, with common side effects including rash and diarrhea.











![Adverse Effects
• Common adverse effects
– Rash (75) [17]
– Diarrhea (54) [18]
– Anorexia (52) [38]
– Dyspnea (41) [35]
– Infection (24) [15]
– Stomatitis (17) [3]
– Pruritus (13) [5]
*(treatment) [placebo]](https://image.slidesharecdn.com/tarcevaok-150308072000-conversion-gate01/75/Tarceva-erlotinib-19-2048.jpg)