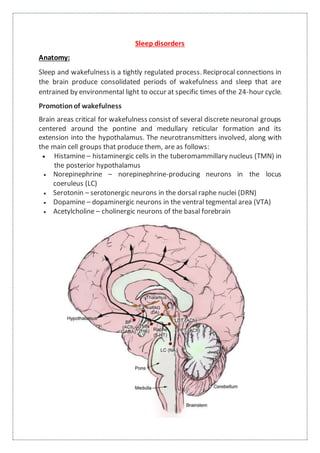
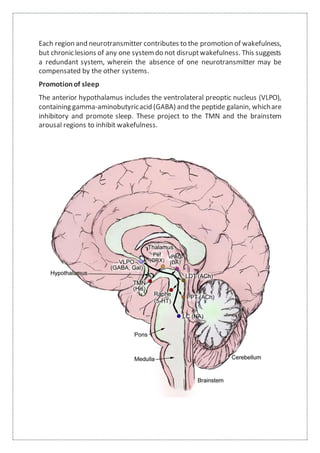
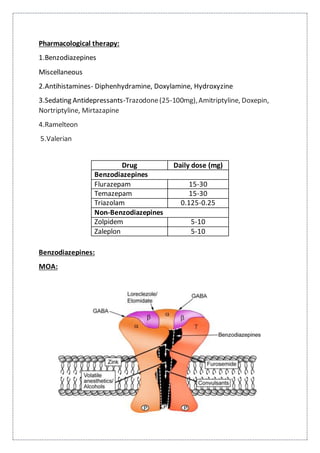
This document summarizes anatomy and neurochemistry related to sleep and wakefulness. It describes brain areas that promote wakefulness like the histaminergic, noradrenergic, serotonergic, dopaminergic, and cholinergic systems. The anterior hypothalamus contains the VLPO which promotes sleep through GABA and galanin projections. Melatonin is produced in the pineal gland under control of the SCN and influences circadian rhythms and sleep. Classification of sleep disorders includes dyssomnias like insomnia, narcolepsy, sleep apnea and circadian disorders as well as parasomnias. Insomnia is defined and classified as transient, acute or chronic. Evaluation and treatment involves identifying