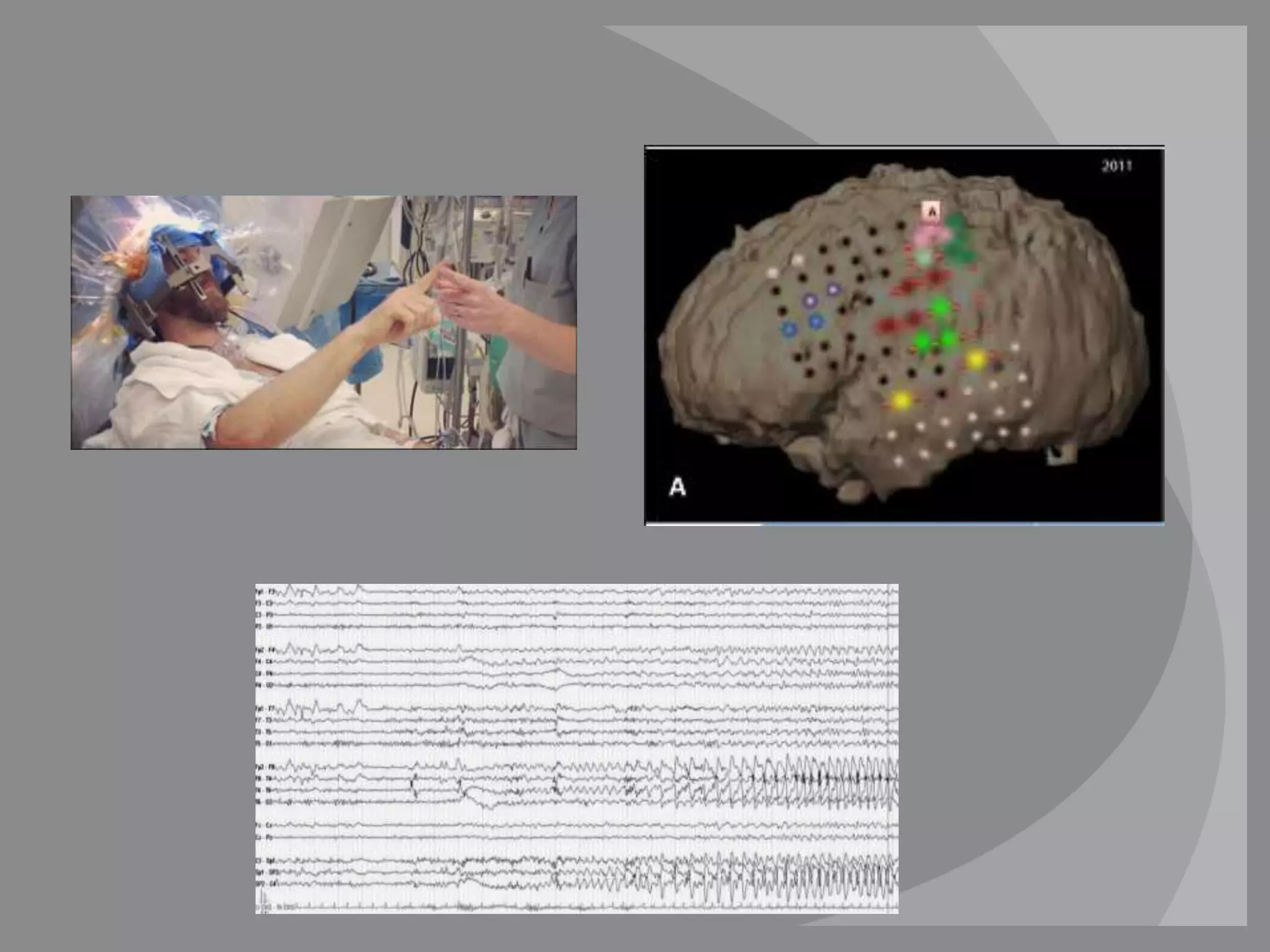
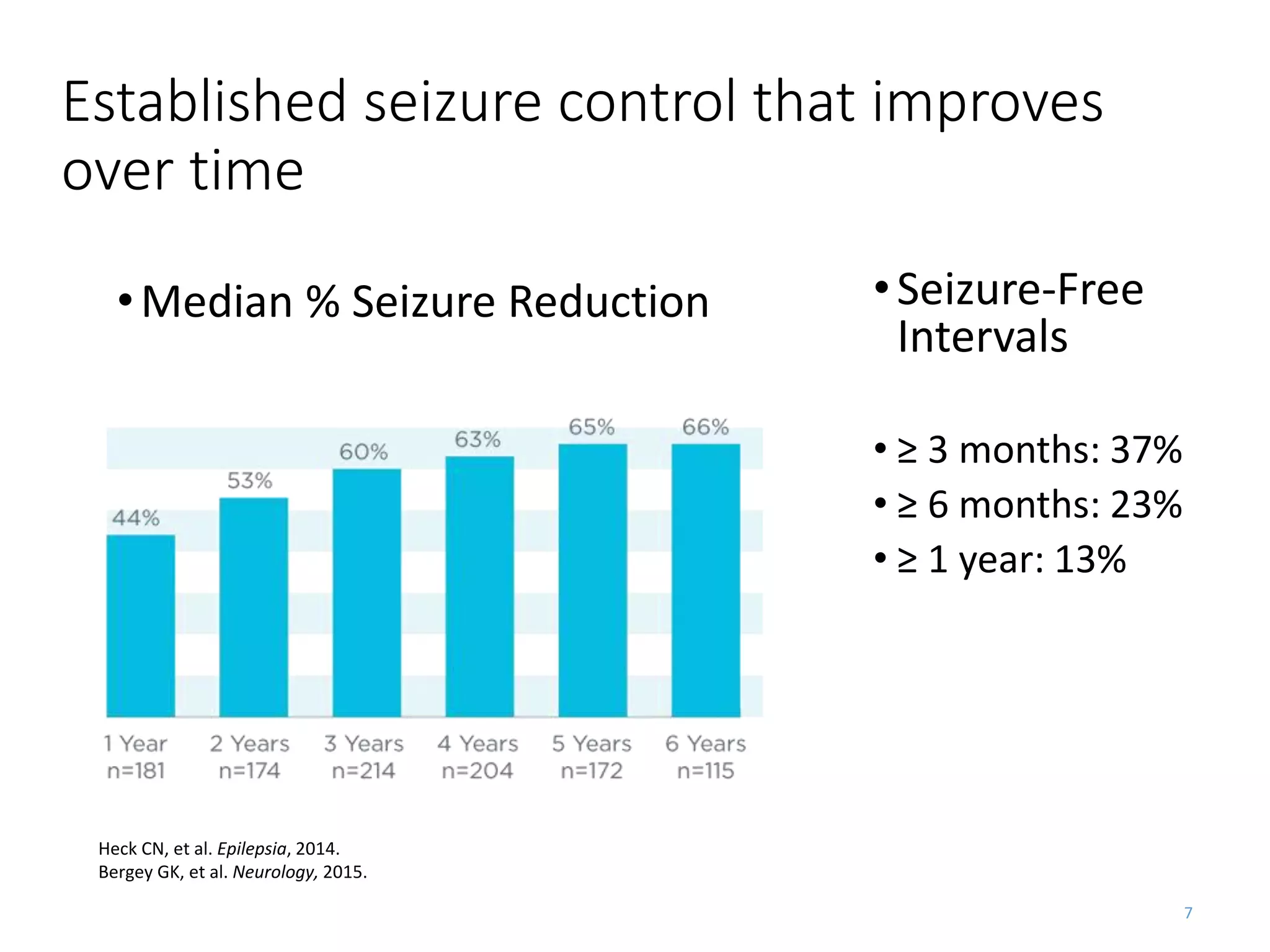
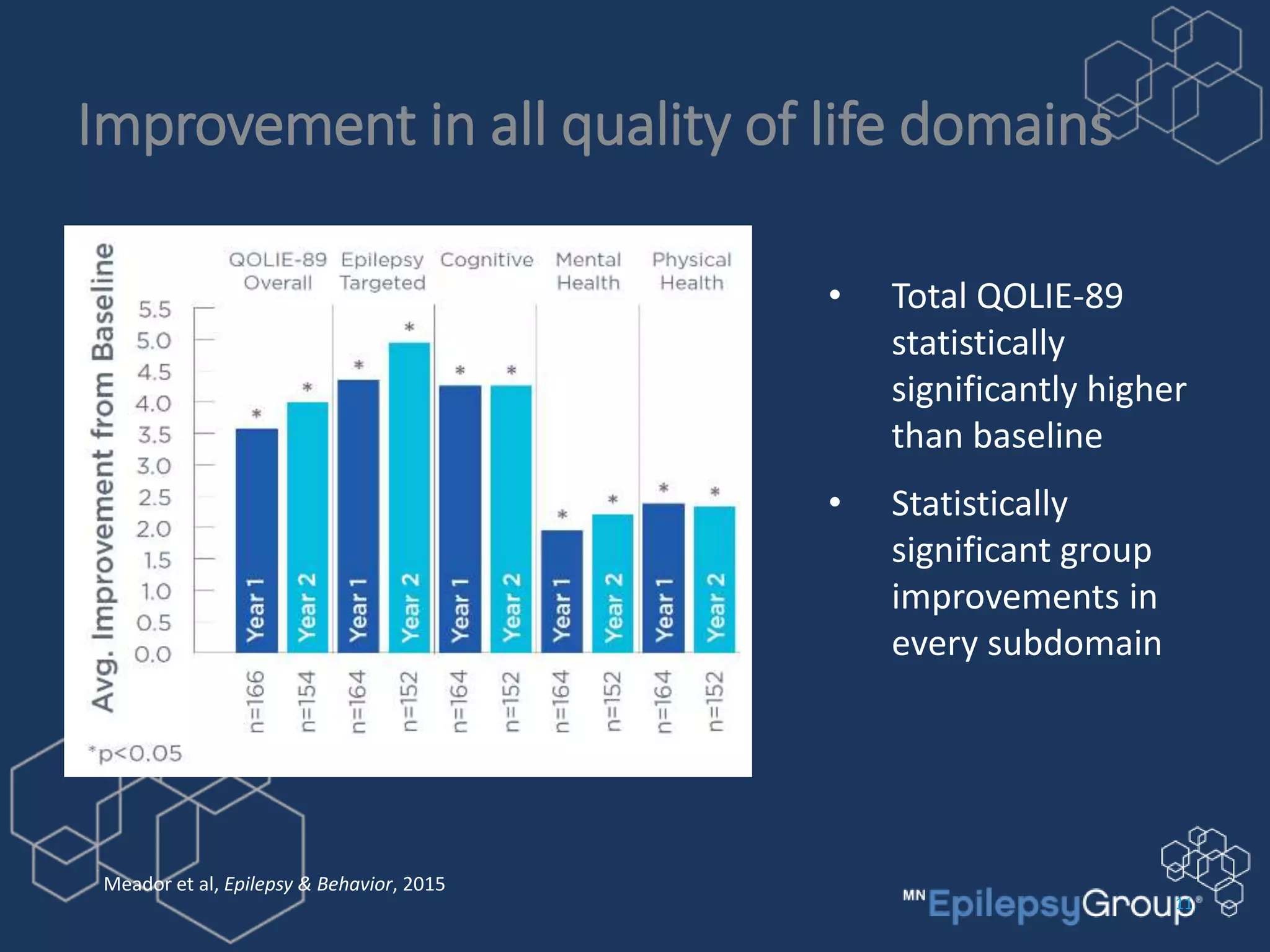
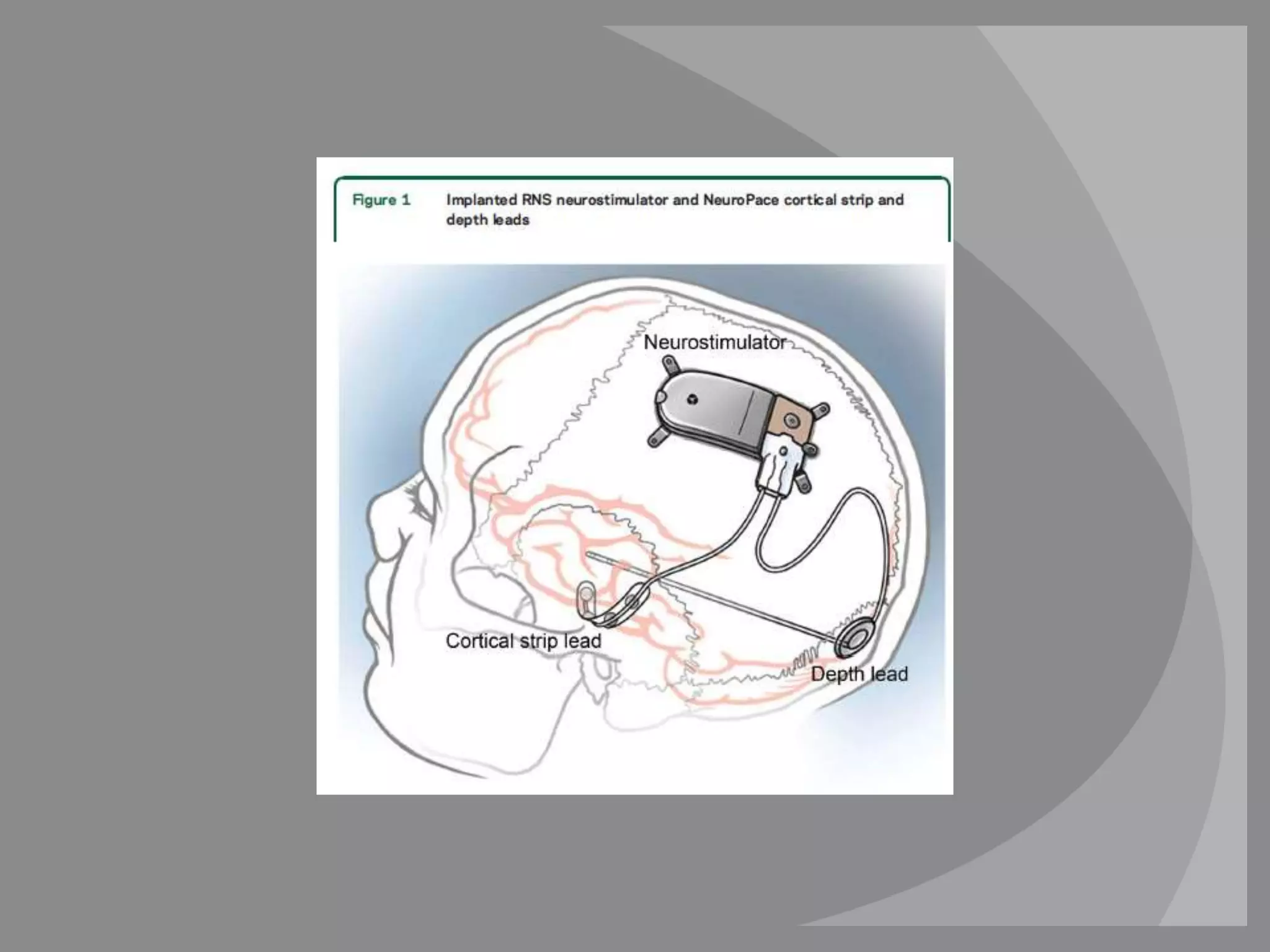
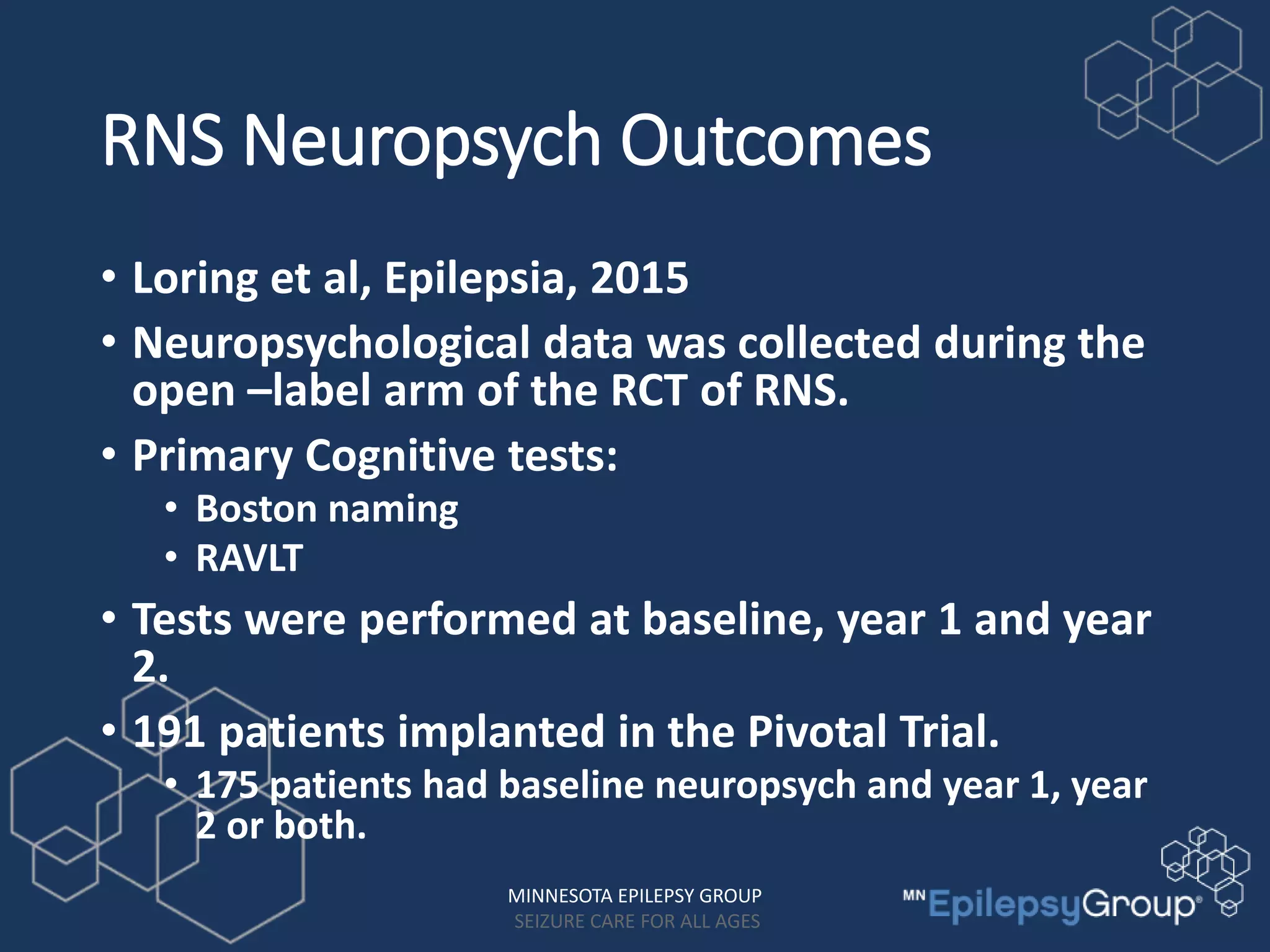
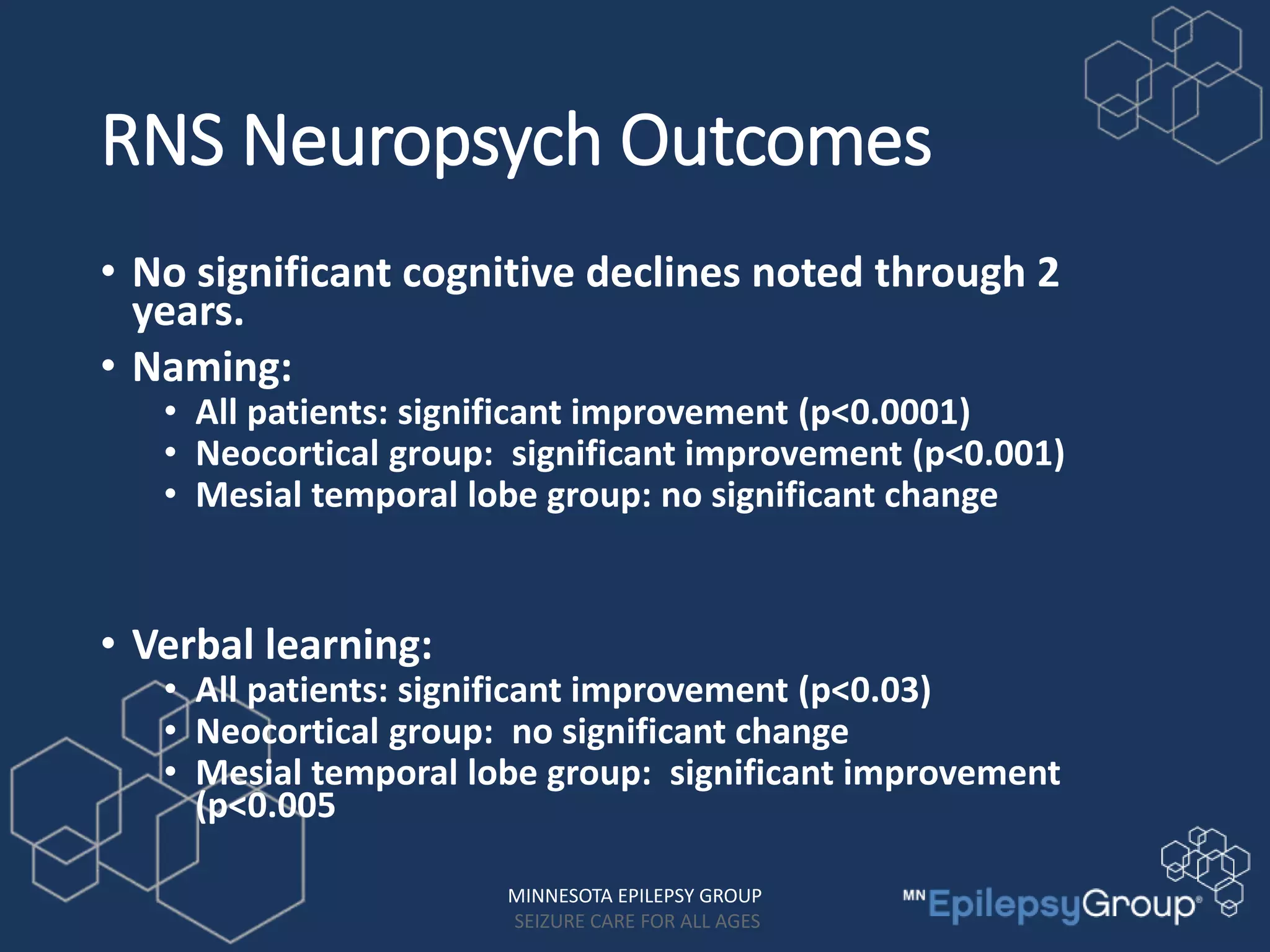
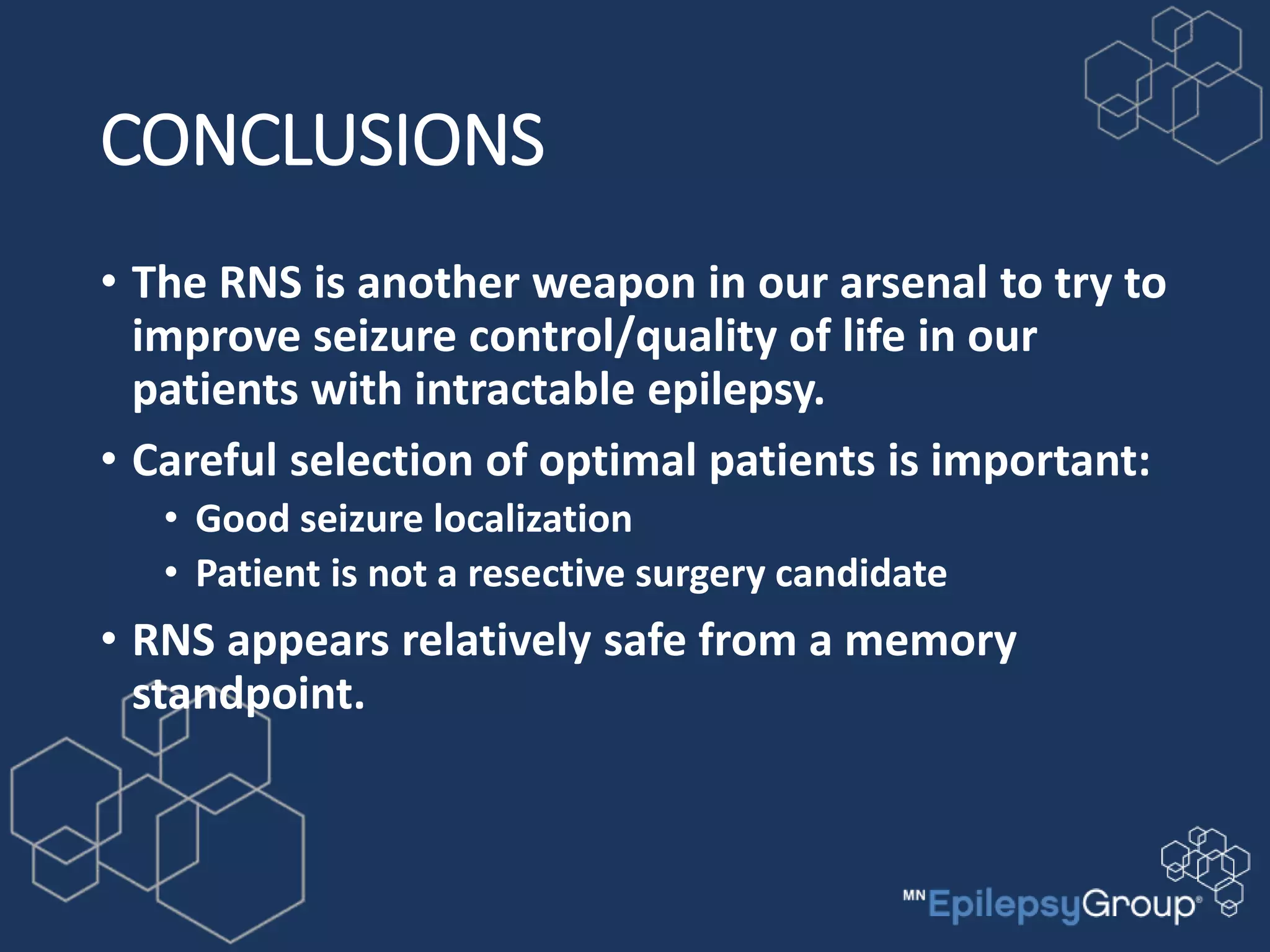
The RNS system is an FDA-approved treatment for drug-resistant epilepsy that involves surgically implanting a neurostimulator device to detect and respond to seizure activity. Clinical trials found that the RNS system provided a median seizure reduction of over 37% within 3 months for treated patients compared to a 25.2% reduction for the sham group. Long-term follow-up over 7 years found the RNS system maintained seizure control effectiveness and was safe, with no chronic neurological effects reported. The RNS system may be an option for patients who have failed two or more anti-seizure medications and are not candidates for resective epilepsy surgery.