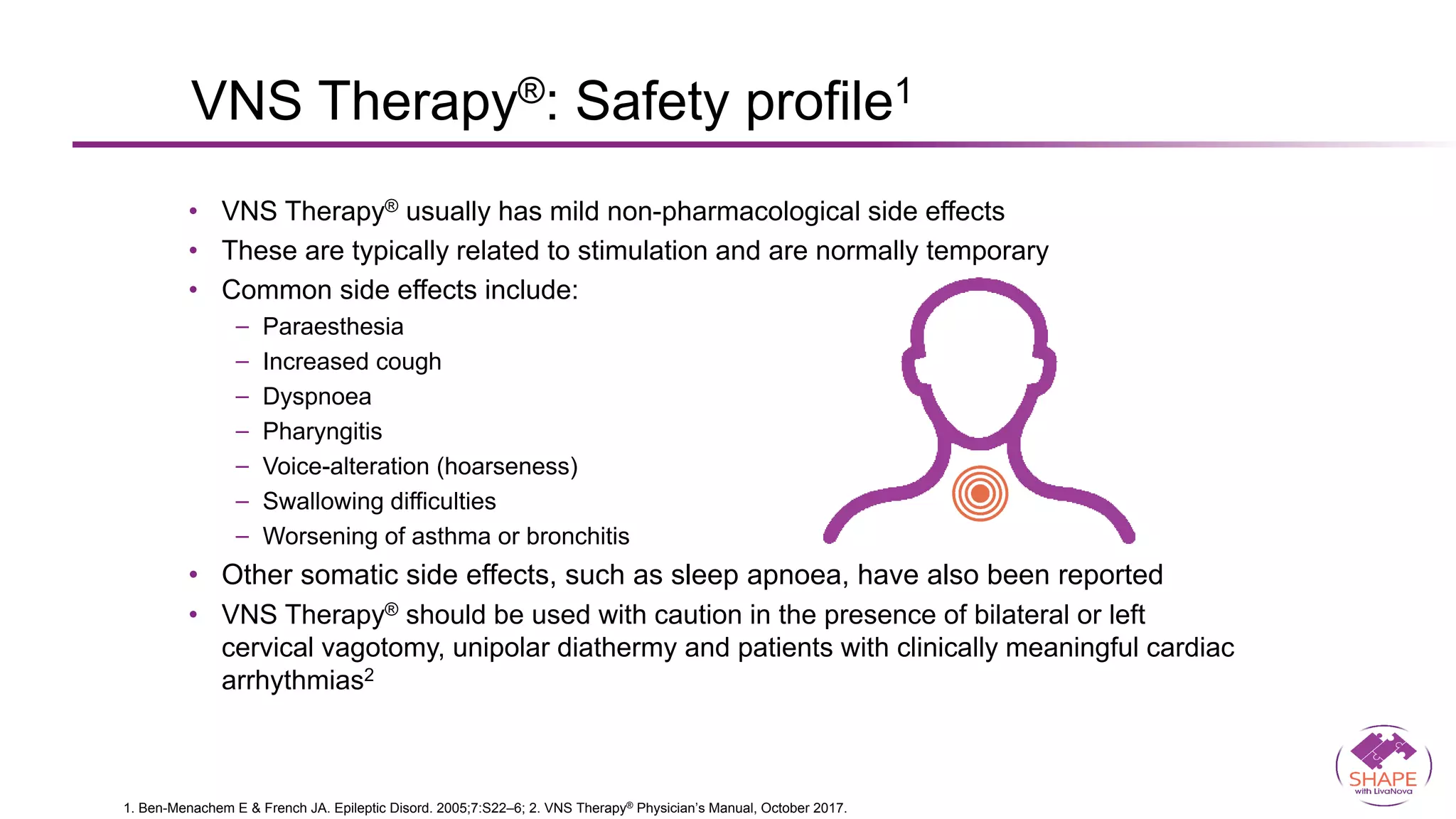
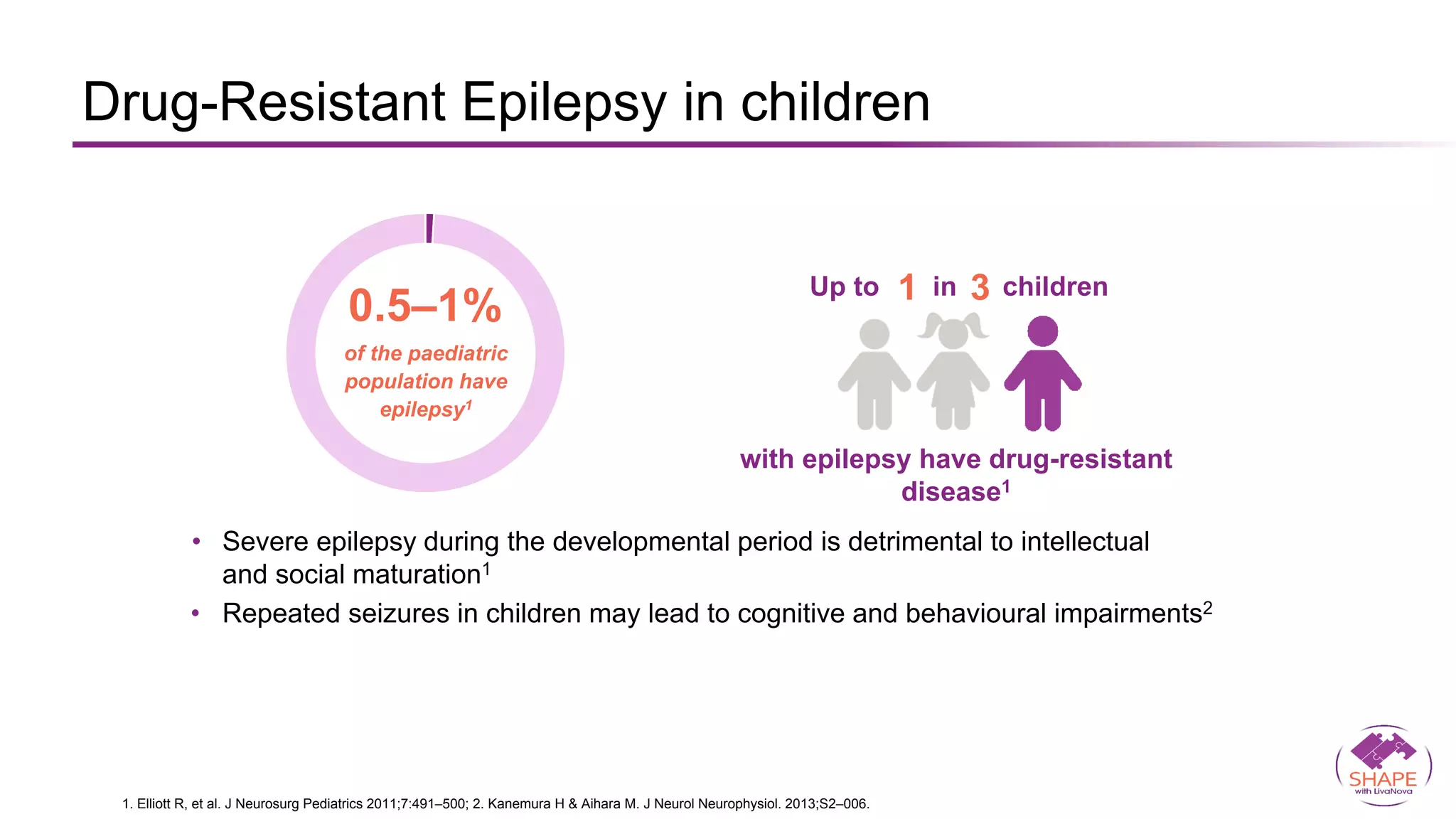
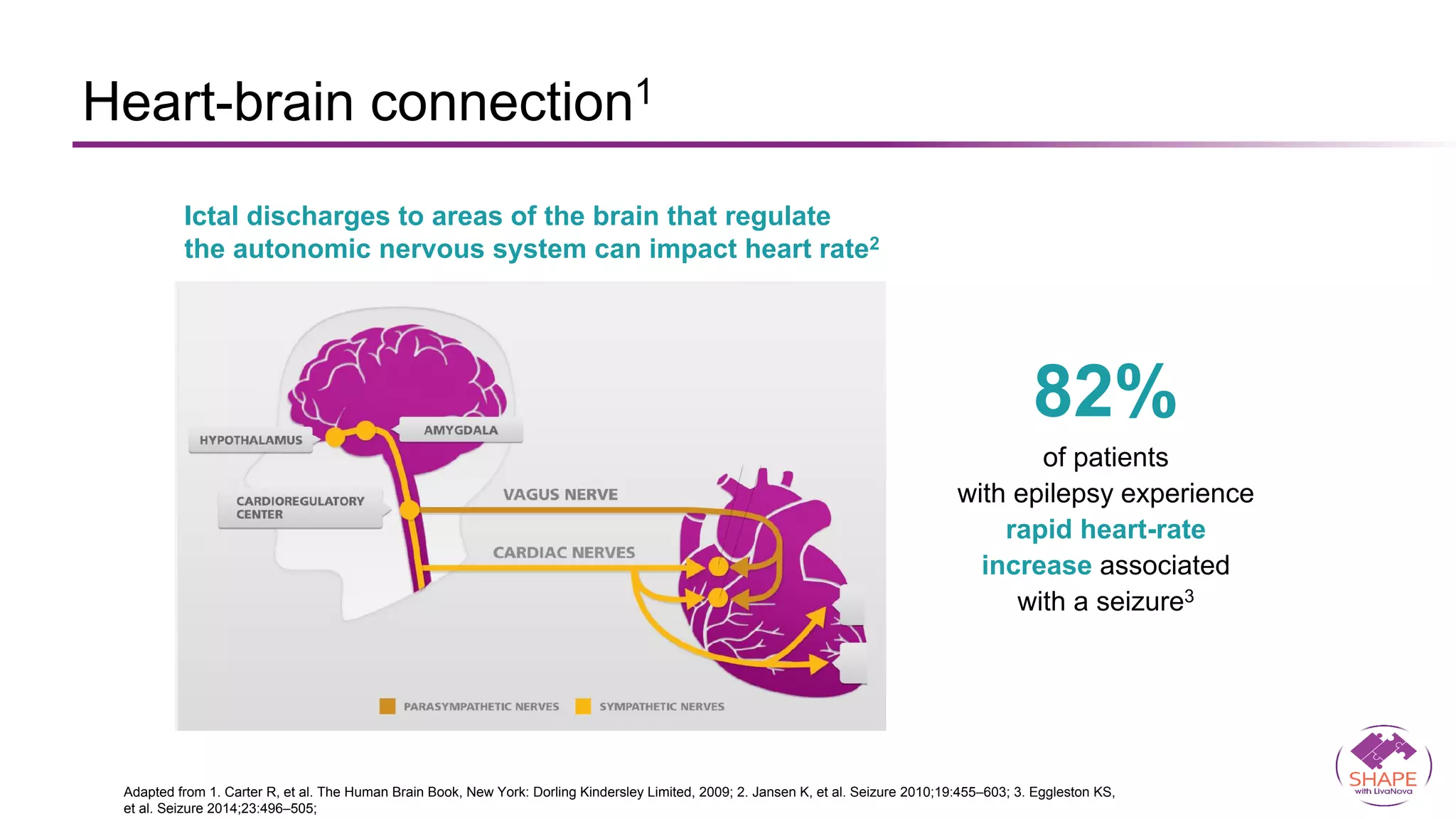
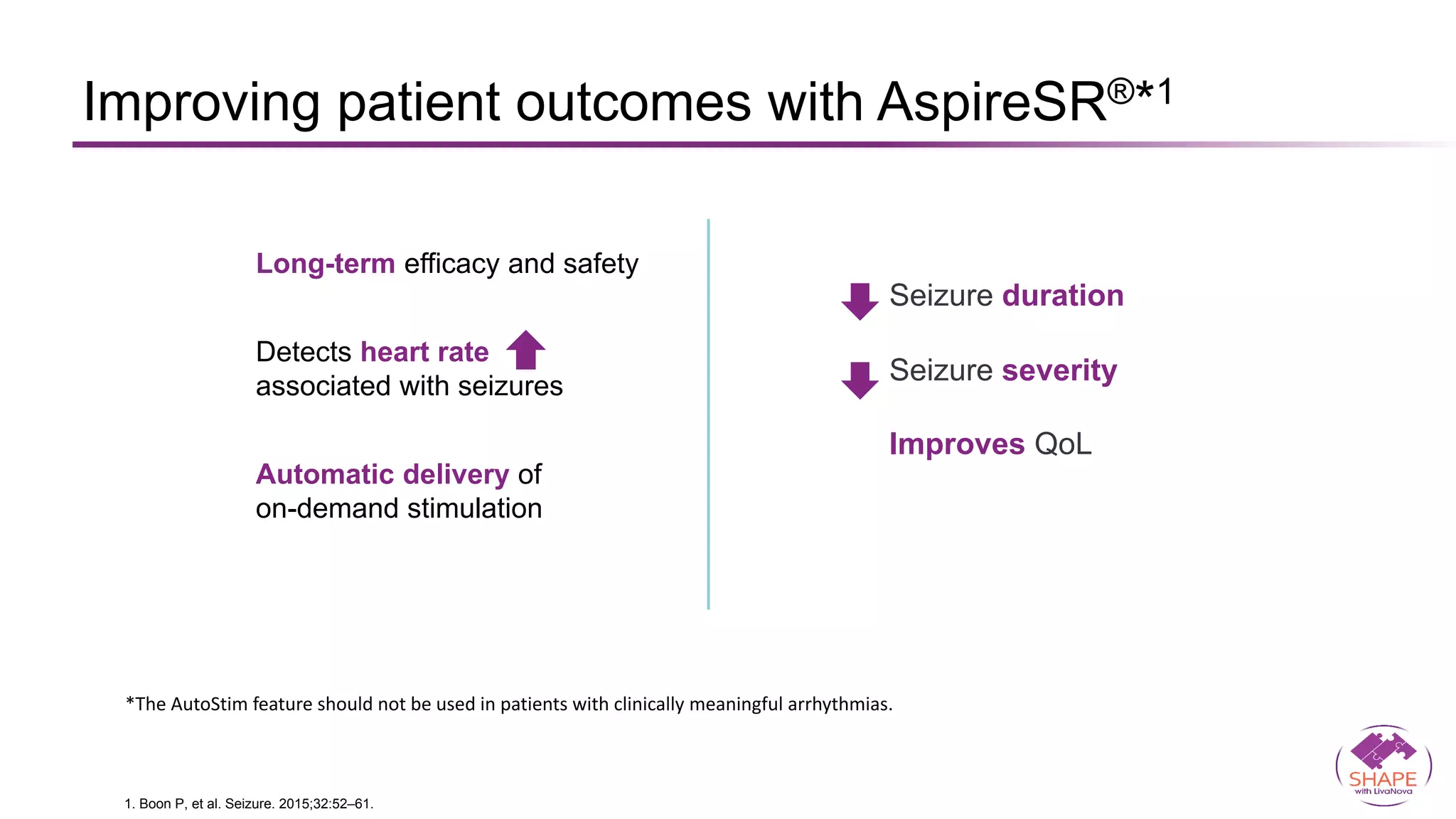
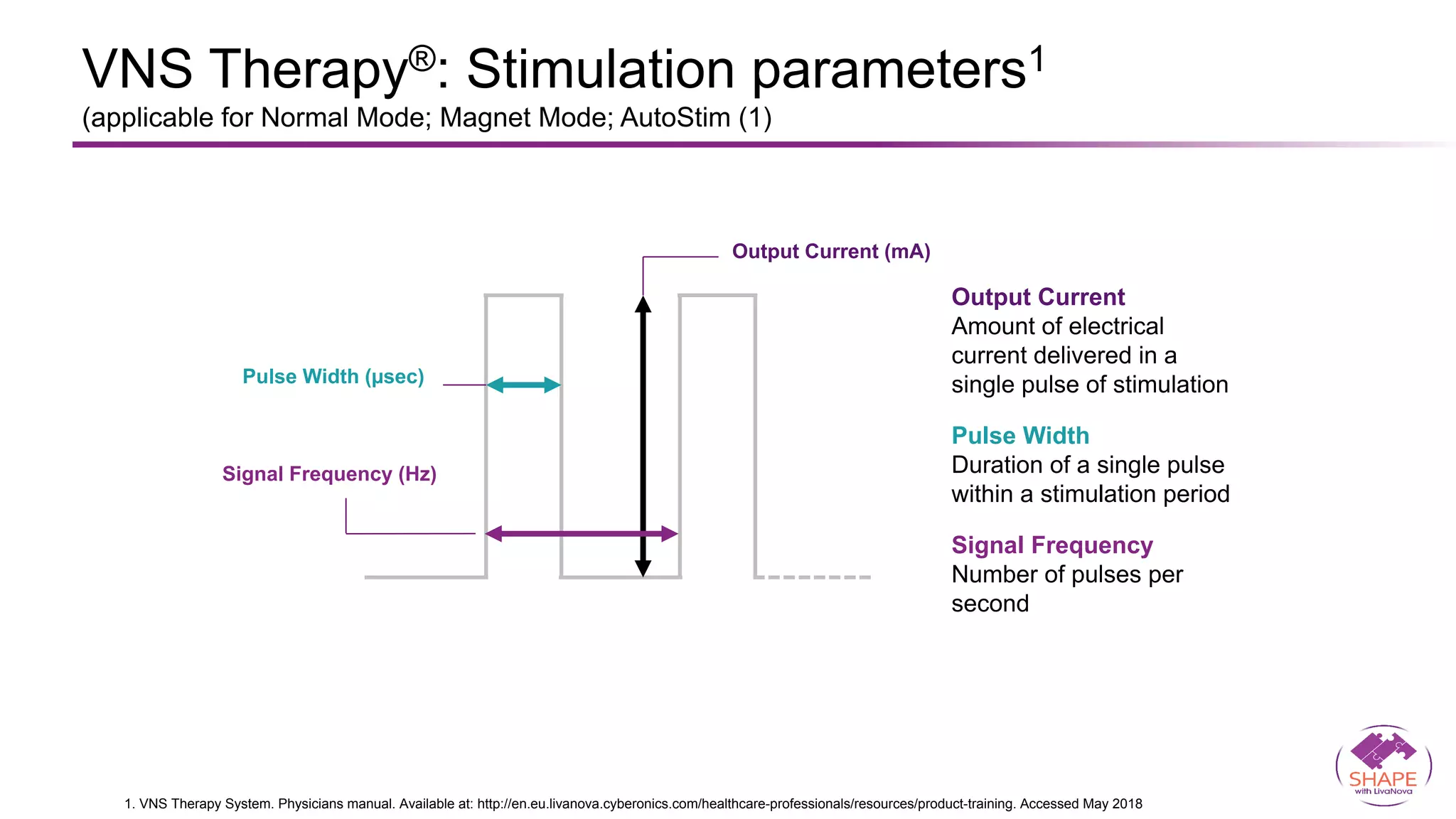
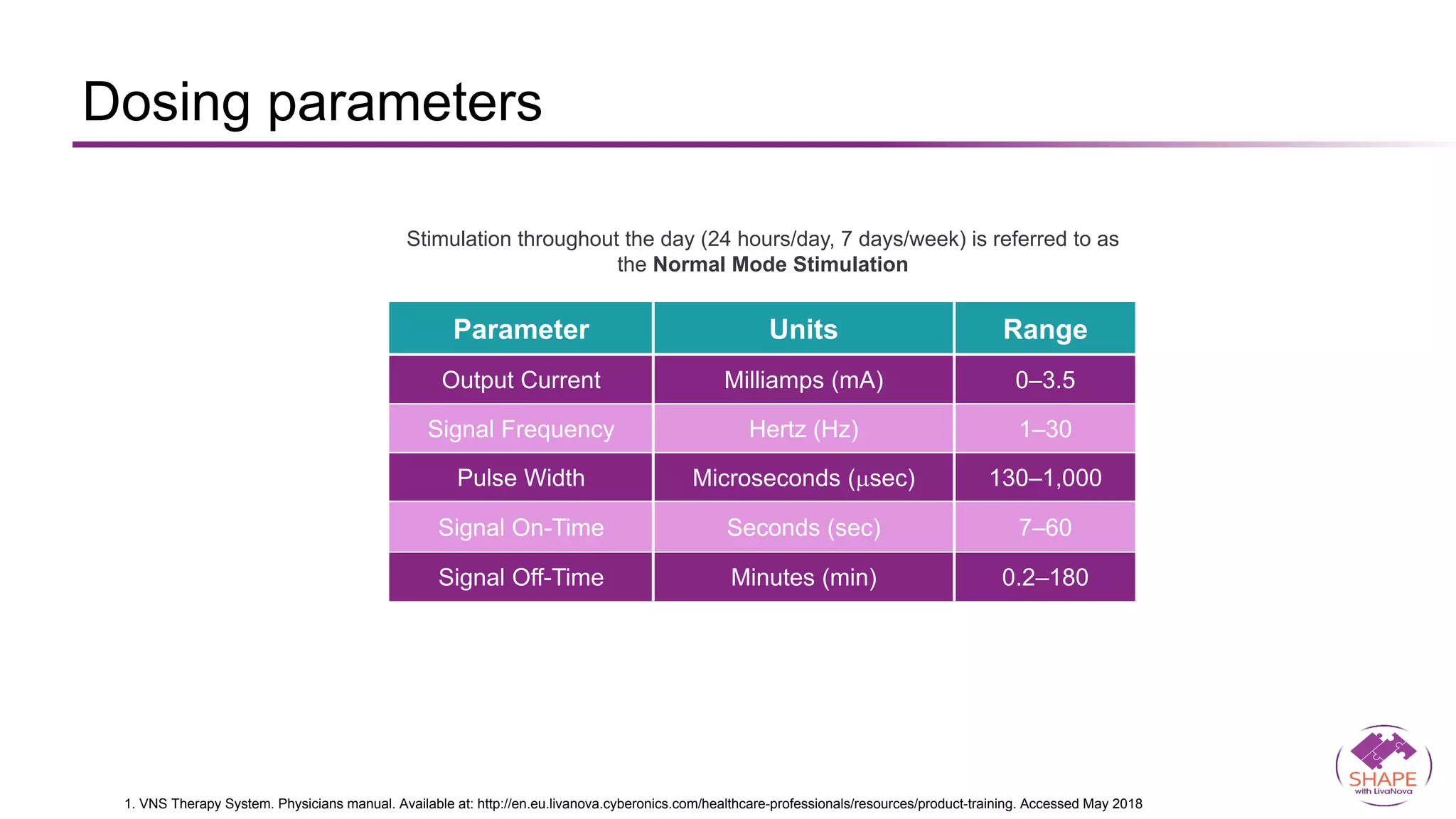
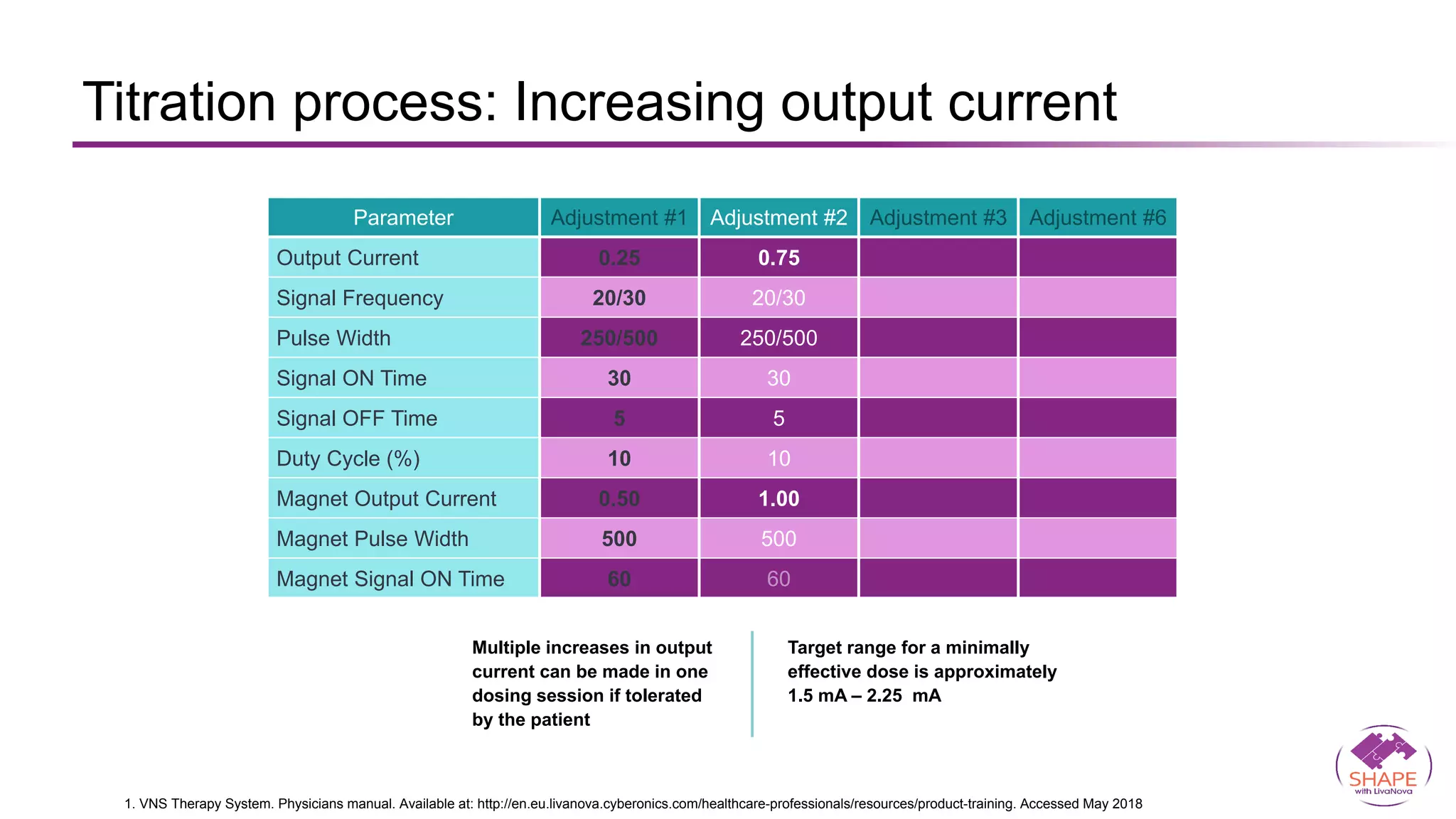
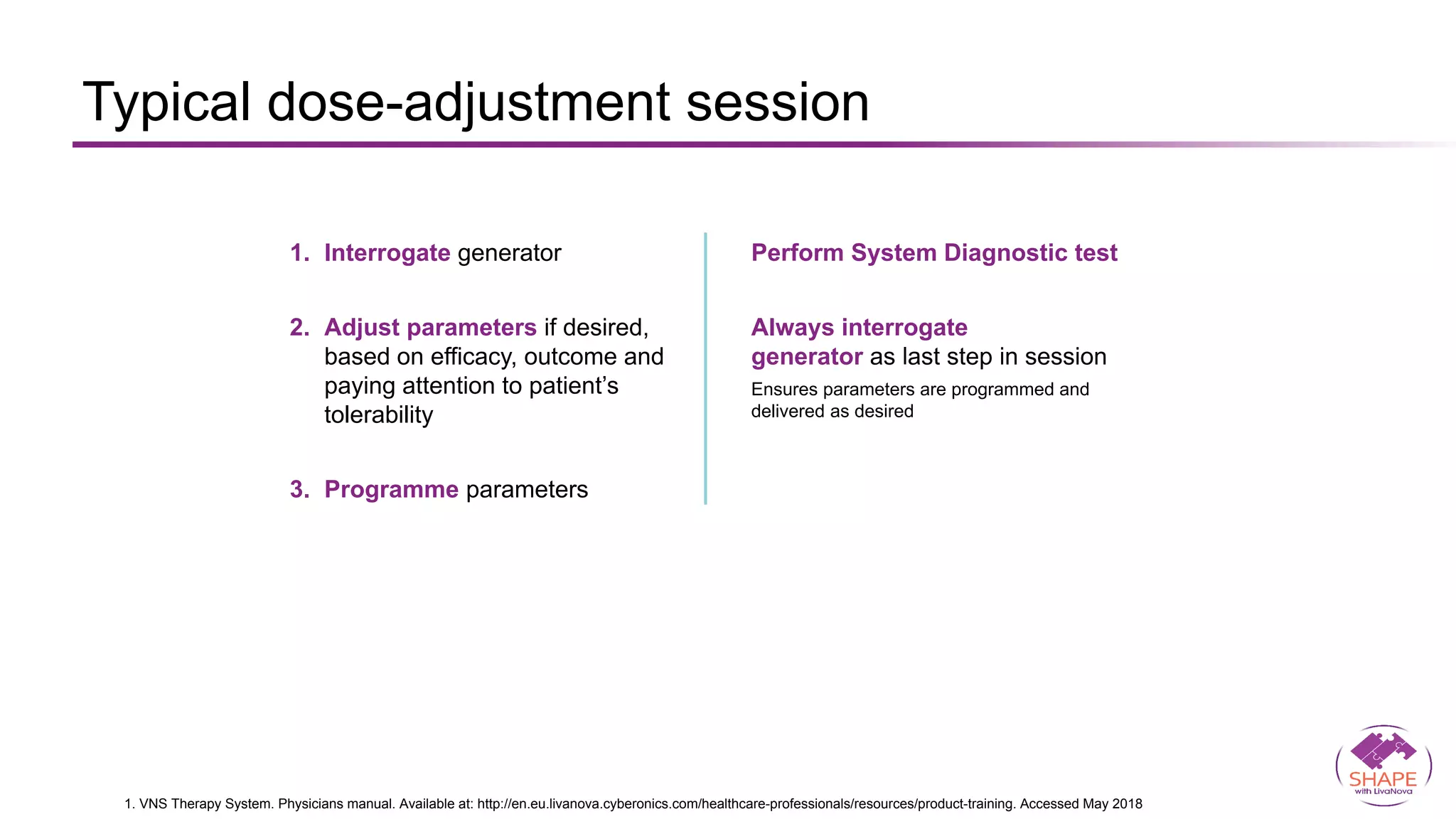
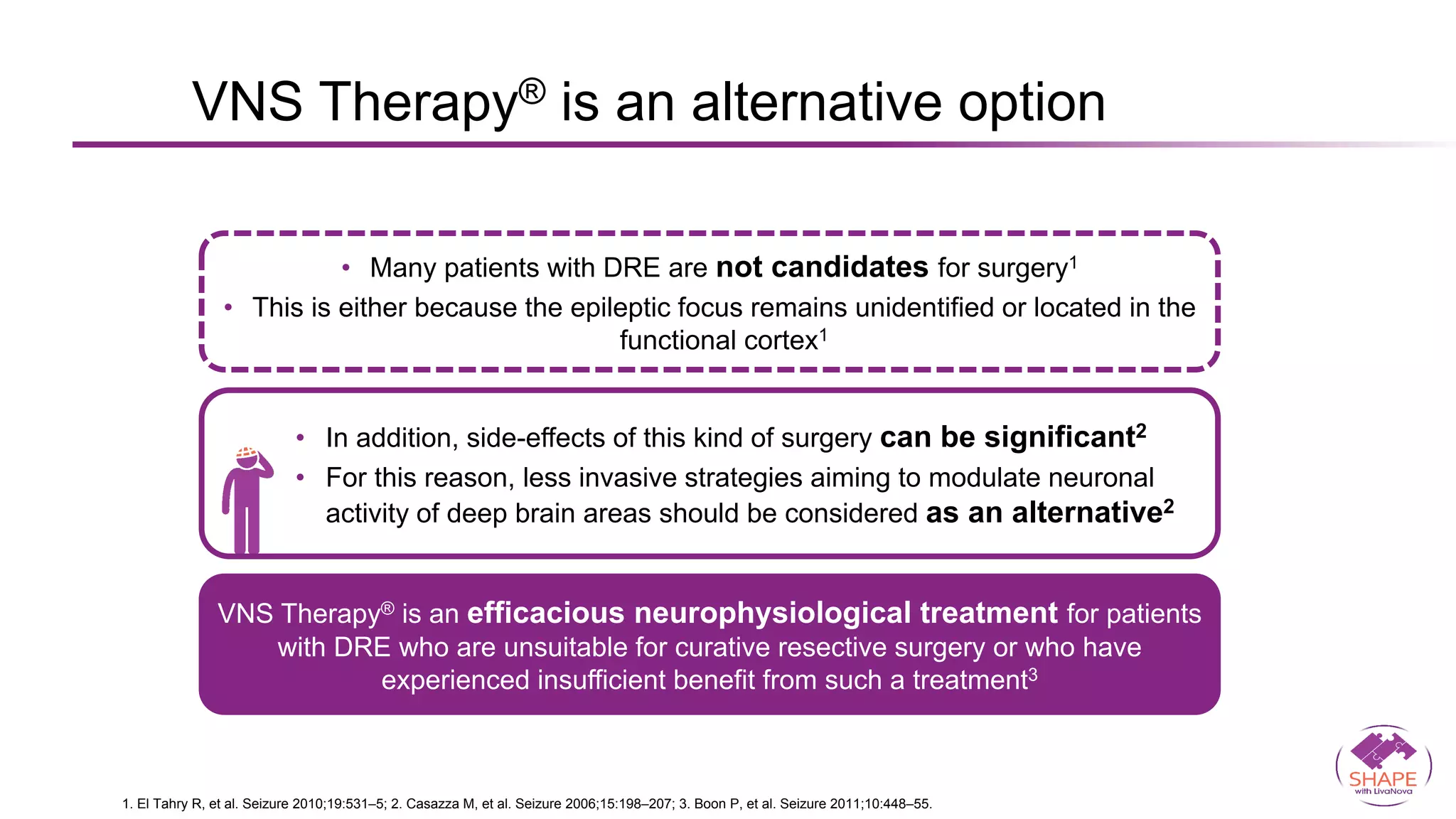
Vagus nerve stimulation (VNS) therapy is an effective adjunctive treatment for patients with drug-resistant epilepsy (DRE) who are not candidates for surgical intervention, offering benefits such as enhanced seizure control, improved quality of life, and a favorable safety profile. The therapy, which involves implantation of a pulse generator connected to the vagus nerve, has shown to reduce seizure frequency and severity while typically presenting mild, temporary side effects. Evidence supports the efficacy of VNS, with many studies highlighting its mechanism of action, including modulation of neurotransmitter expression and increased cerebral blood flow.











![DRE patients
after 1 year of VNS Therapy® (n=10)
DRE patients
eligible for VNS Therapy® (n=7)
-20 0 20 40 60 80 100
GRD distribution (% change)
Seizure
frequency
reduction
GRD distribution (% change)
Seizure
frequency
reduction
-20
-5
10
25
40
55
70
85
-10 0 10 20 30
-15
-5
5
15
25
35
Changes in cortical GABAA receptor density (GRD) measured
by SPECT with the GABAA receptor agonist [123I] iomazenil
• Clinical efficacy of
VNS Therapy®
correlates with up-
regulation of GRD
in DRE
• GABAA receptor-
mediated neuronal
inhibition is
enhanced by VNS
Therapy®1
Adjunctive VNS Therapy® modulates GABAA
receptor expression1
Adapted from 1. Marrosu F, et al. Epilepsy Res. 2003;55:59–70.
SPECT=single-photon emission computed tomography
GRD : GABA receptor denisty.](https://image.slidesharecdn.com/vnslong-210508210155/75/Vagal-Nerve-stimulation-14-2048.jpg)