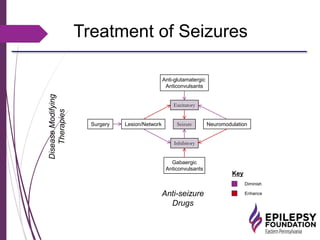
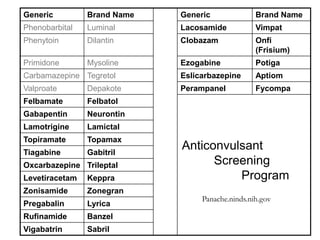
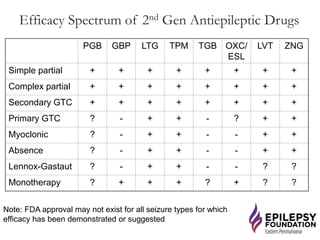
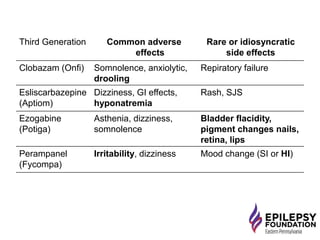
The document discusses advancements in epilepsy treatment, including various anti-seizure medications and clinical trials for drug-resistant epilepsy. It highlights the efficacy spectrum of different generations of antiepileptic drugs and ongoing trials for new treatments such as intranasal midazolam and the RNS® system. Additionally, it reviews surgical options and the long-term effectiveness and safety of devices like VNS and RNS in reducing seizure frequency.













![Transorbital endoscopic
amygdalohippocampectomy
Minimally invasive
Advantage is that cortex
spared
Procedure offered only
at Penn by Dr. Lucas
Transorbital endoscopic
amygdalohippocampectomy: a feasibility
investigation. Source:
Journal of neurosurgery [0022-3085] Chen, H I
yr:2014 vol:120 iss:6 pg:1428 -1436](https://image.slidesharecdn.com/kdaviseftalk112014updated2015-150708144141-lva1-app6891/85/New-Treatment-Devices-and-Clinical-Trials-18-320.jpg)



















