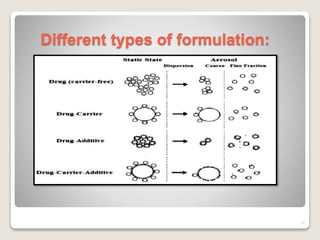
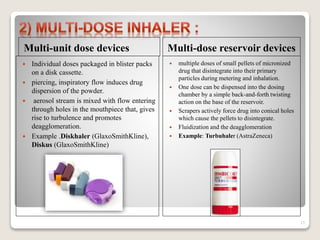
Dry powder inhalers (DPIs) deliver dry powder drug formulations for local or systemic effects via the pulmonary route. DPIs are propellant-free and provide rapid drug action with high doses and reproducibility. For systemic effects, particle sizes less than 2 μm are needed for deposition in small airways. However, deposition efficiency depends on patient inhalation and larger particles have greater potential for dose variability. Future research aims to improve deposition and achieve intracellular delivery of complex drugs using DPIs.