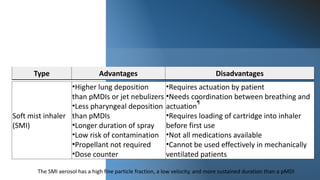
The document provides a history of inhalation therapy and devices used for inhalation of medications. It discusses the earliest known uses of inhalation from 2000 BC to the first inhaler device created in 1778. The document then summarizes the main types of modern inhalation devices including metered dose inhalers, dry powder inhalers, soft mist inhalers, and nebulizers. It describes the mechanisms and key features of each device type.





















































![Special Situations
• Acute asthma exacerbation — Two issues that arise when choosing an aerosol delivery system for bronchodilator
medication during exacerbations of asthma are whether to use an MDI or a nebulizer and whether to use continuous or
intermittent nebulization for hospital based treatment. These choices are typically based on the severity of the
exacerbation and also clinician and patient preference (algorithm 1). For patients who have an asthma exacerbation that is
mild to moderate in severity (eg, mild to no dyspnea at rest and peak expiratory flow ≥40 percent of predicted),
administration of the beta 2-agonist albuterol via a pMDI (2 to 6 puffs for treatment at home, 4 to 8 puffs for emergency
room or hospital treatment) combined with a spacer or chamber device (eg, Aerochamber, Optichamber Diamond, Vortex)
results in comparable improvements in lung function compared to nebulizer delivery, although the actual dose delivered
by a pMDI is much lower (table 2). Similar results with pMDIs have been reported in patients with severe exacerbations,
but only a small number of such patients have been studied. Generally, nebulizer treatments (every 20 minutes or
continuous) are preferred for more severe asthma exacerbations. (See "Acute exacerbations of asthma in adults:
Emergency department and inpatient management", section on 'Nebulizer versus MDI'.) For patients with severe asthma
exacerbations (eg, dyspnea at rest, accessory muscle use, retractions, forced expiratory volume in one second or peak
expiratory flow <40 percent predicted), beta agonists are often administered continuously (eg, albuterol 5 to 15 mg/hour)
rather than intermittently [16,88,89]. This method of bronchodilator administration is equally effective compared to
frequent intermittent nebulization [8,90]. Several studies have established the safety of continuous nebulization, even
when high doses (eg, 20 mg/hour of albuterol) are used [42,88,91]. However, continuous nebulization of albuterol (10
mg/hour) in healthy adults has been associated with a decrease in serum potassium of 0.5 mEq/L (95% CI: -0.72 to -0.28
mEq/L), which could be clinically important in patients with a low potassium level prior to therapy [92]. Continuous
nebulization may be most beneficial in patients with the most severe pulmonary dysfunction [88]. The specialized delivery
systems adapted for continuous nebulization are described above. (See 'Continuous nebulization' above.)](https://image.slidesharecdn.com/nebulisationmedications-220910105048-ab6bea76/85/Nebulisation-Medications-pptx-57-320.jpg)
![• Metered dose inhaler — A special actuator is needed to adapt the pMDI
into the ventilator circuit (picture 7) [107,108]. The size, shape, and design
of these actuators have a major impact on drug delivery to the patient. A
pMDI with a chamber results in a four- to six-fold greater delivery of
aerosol than MDI actuation into a connector attached directly to the
endotracheal tube, or into an in-line device that lacks a chamber [98].
When using a pMDI during mechanical ventilation, it is important to
synchronize actuation with inspiratory airflow to optimize drug delivery.
Properly used, a pMDI may deliver a more consistent dose than a nebulizer
[109]. The following technique has been proposed for using pMDIs in
mechanically ventilated adult patients [110]: Revefenacin - (Yupelri)
inhalation solution is the only once-daily, nebulized bronchodilator for
maintenance treatment of COPD [85,86]. Revefenacin is a LAMA and is
nebulized with a standard jet nebulizer connected to a compressor. (See
"Role of anticholinergic therapy in COPD", section on 'Revefenacin'.)](https://image.slidesharecdn.com/nebulisationmedications-220910105048-ab6bea76/85/Nebulisation-Medications-pptx-58-320.jpg)
![• Amikacin – Amikacin (Arikayce) liposome inhalation suspension is delivered once daily with the Lamira mesh nebulizer system.
During nebulization, approximately 70 percent of the amikacin dose remains encapsulated within liposomes while approximately
30 percent of the dose is released as free amikacin. Nebulized amikacin is indicated only for patients with refractory disease
caused by Mycobacterium avium complex (MAC) who have limited or no alternative treatment options [87]. (See "Treatment of
Mycobacterium avium complex lung infection in adults", section on 'Efficacy of alternative agents'. ● Shake the pMDI vigorously
• ● Place canister in the actuator of a cylindrical spacer situated in the inspiratory limb of ventilator circuit (picture 7)
• ● Actuate the pMDI once only with the onset of inspiration by the ventilator
• ● Repeat actuations after 15 seconds until the total dose is delivered
• Helium-oxygen mixtures affect aerosol deposition, and in vitro modeling has reported a 50 percent increase in deposition of
albuterol from a pMDI during mechanical ventilation when heliox was used as the driving gas [111]. However, heliox can interfere
with the functioning of flow sensors and oxygen levels when delivered through some ventilators, and care must be taken if this
approach is employed with a ventilator that is not approved for heliox administration [112-114]. (See "Physiology and clinical use
of heliox", section on 'Instrument recalibration'.)
• The use of a heat and moisture exchanger (HME) in the ventilator circuit can filter out the aerosol when a pMDI (or nebulizer) is
used. Commercially available devices can be used to bypass the HME when a pMDI is used (picture 8 and picture 9). Alternatively,
the HME must be removed from the circuit when the aerosol is delivered [115].](https://image.slidesharecdn.com/nebulisationmedications-220910105048-ab6bea76/85/Nebulisation-Medications-pptx-59-320.jpg)
![• Nebulizer — The optimal methods for delivery of nebulized medication to mechanically ventilated patients are not well-
established. Delivery of a large tidal volume, use of an end-inspiratory pause, and use of a slow inspiratory flow affect aerosol
delivery by jet nebulizer but not by a pMDI [95].
• Nebulizer performance can be optimized by placing the nebulizer 30 cm from the endotracheal tube, rather than at the Y-piece,
because the inspiratory ventilator tubing acts as a spacer. In a simulation model, delivery of albuterol via mesh nebulizer was two
to four times greater than with a jet nebulizer, and placement of the mesh nebulizer in the ventilator tubing on the ventilator side
of the humidifier, rather than closer to the patient, increased drug delivery [100].
• Operating the nebulizer only during inspiration is more efficient for aerosol delivery compared with continuous aerosol generation
throughout the respiratory cycle. When a breath-actuated nebulizer is used, the delivered dose increases by more than five-fold.
In addition, when the humidifier is bypassed the delivered dose increases by a factor of nearly four [96].
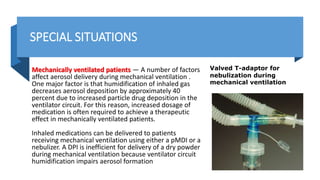
• Disadvantages of jet nebulizer use during mechanical ventilation include circuit contamination due to interrupting the ventilator
tubing circuit, decreased ability of the patient to trigger the ventilator, and the associated increases in tidal volume and airway
pressure due to nebulizer flow. Valved T-piece devices are commercially available and commonly used to allow the nebulizer to be
inserted within the ventilator circuit without disconnecting the patient from the ventilator, thus avoiding interruption of
mechanical ventilation for nebulizer insertion and removal
• The mesh nebulizer can be used effectively during mechanical ventilation and is placed between the ventilator outlet and the
heated humidifier Unlike the jet nebulizer, the mesh nebulizer remains in the ventilator circuit and does not interfere with
ventilator function (eg, no additional gas flow, no effect on triggering). (See 'Mesh nebulizers' above.)](https://image.slidesharecdn.com/nebulisationmedications-220910105048-ab6bea76/85/Nebulisation-Medications-pptx-60-320.jpg)
![• Choice of device — Although the jet nebulizer is less efficient than the pMDI during
mechanical ventilation, the nebulizer can deliver a greater cumulative dose to the lower
respiratory tract [117]. Thus, nebulizers and pMDIs produce similar therapeutic effects in
mechanically ventilated patients [118]. The use of a pMDI for routine bronchodilator
therapy in ventilator-supported patients has been preferred because of the problems
associated with the use of nebulizers, including contamination and triggering difficulty, as
well as increased pressure and volume delivery. However, use of a mesh nebulizer avoids
several of the problems of the jet nebulizer and performs comparably to pMDIs.
Compared with the pMDI, the mesh nebulizer is a convenient and efficient delivery
method in mechanically ventilated patients [119].
• Aerosol delivery by pMDI is easy to administer, involves less personnel time than a
nebulizer, provides a reliable dose of the drug, and is free from the risk of bacterial
contamination. When a pMDI is used with an in-line spacer, the ventilator circuit does
not need to be disconnected with each treatment; this may reduce the risk of ventilator-
associated pneumonia. This also prevents the loss of positive end-expiratory pressure
(PEEP) in patients with acute lung injury (ALI) and acute respiratory distress syndrome
(ARDS).](https://image.slidesharecdn.com/nebulisationmedications-220910105048-ab6bea76/85/Nebulisation-Medications-pptx-61-320.jpg)
![• Patients using high flow nasal cannula — The high flow nasal cannula is
increasingly used for hypoxemic respiratory failure and can also be used for
aerosol delivery in the intensive care unit (ICU) [126,127]. The results of in
vitro studies suggest that aerosols can be delivered by HFNC, and there is
anecdotal experience suggesting benefit [65]. At high flows, the amount of
aerosol delivery is likely to be very low [65,66]. In one study, pulmonary
drug delivery through the high-flow nasal cannula was about 1 to 4 percent
of the amount placed in the nebulizer, with a higher efficiency for a mesh
nebulizer than a jet nebulizer [17]. However, in a separate study of 26
subjects with COPD, the physiologic response to inhaled bronchodilator
was similar for mouthpiece and nasal cannula at a flow of 35 L/minute
[128]. The lower deposition with high flow nasal cannula might also be
overcome by increasing the dose [65]. A pMDI, SMI, or DPI device cannot
be used with high flow nasal cannula](https://image.slidesharecdn.com/nebulisationmedications-220910105048-ab6bea76/85/Nebulisation-Medications-pptx-62-320.jpg)
















