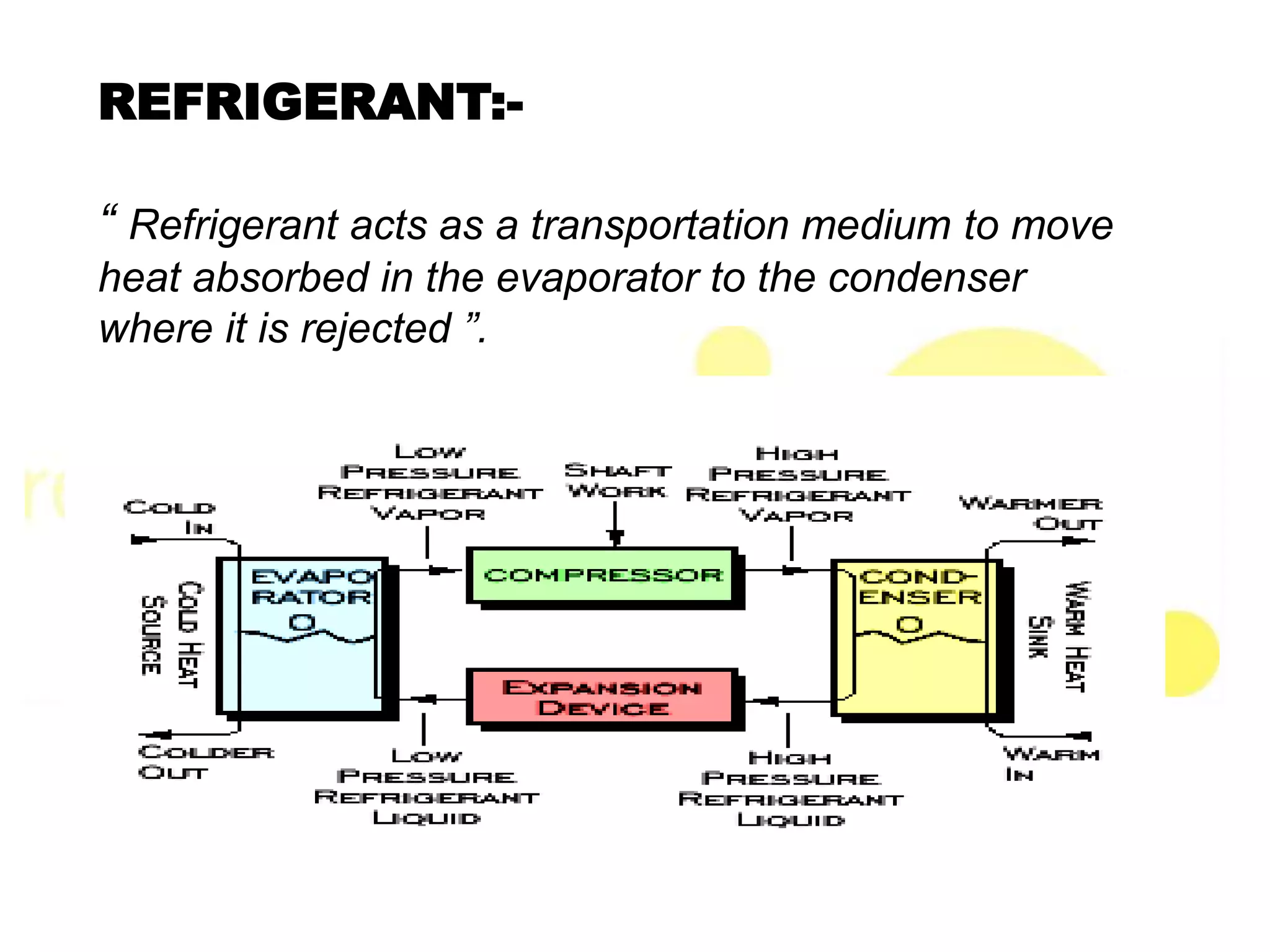
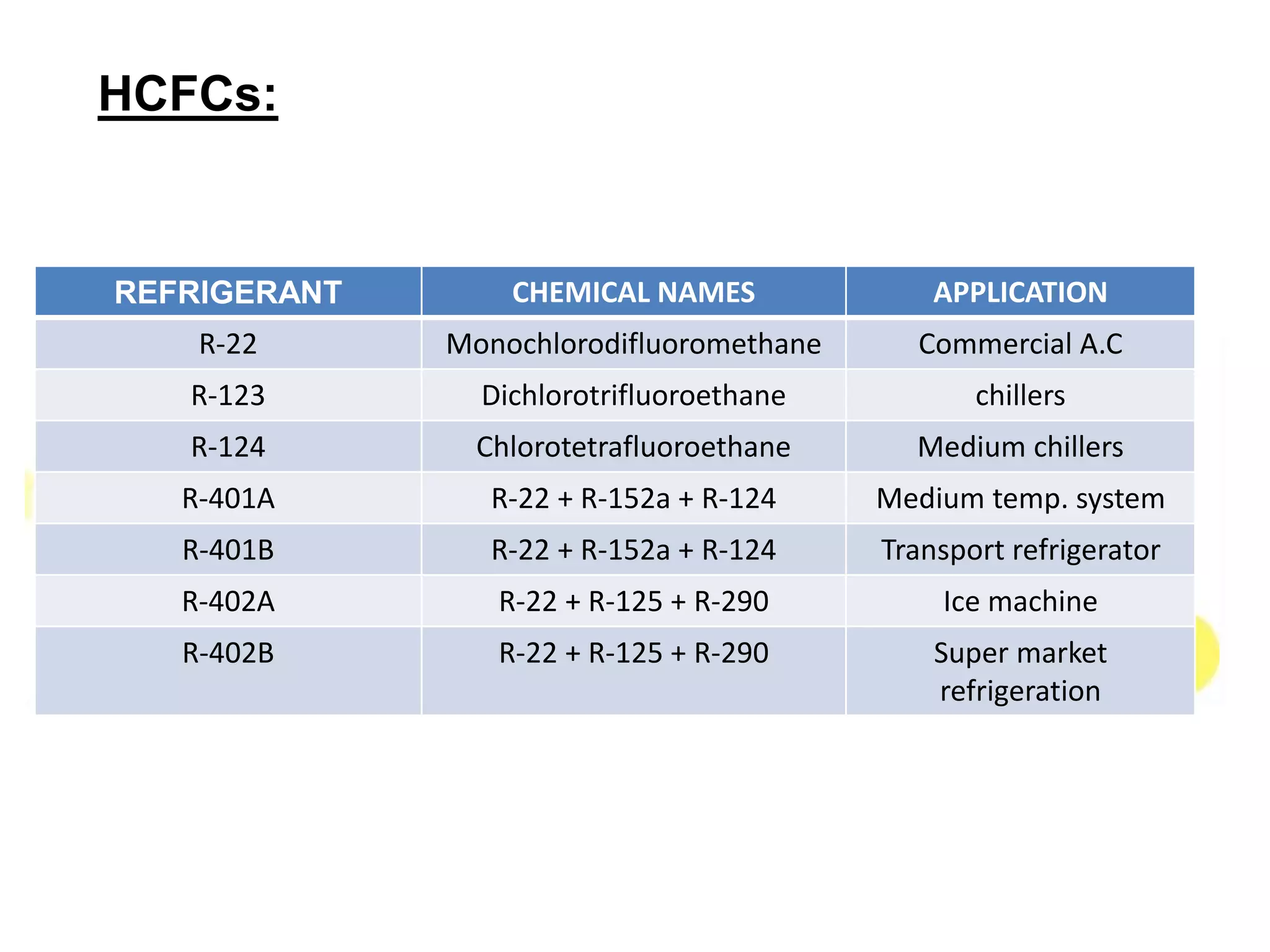
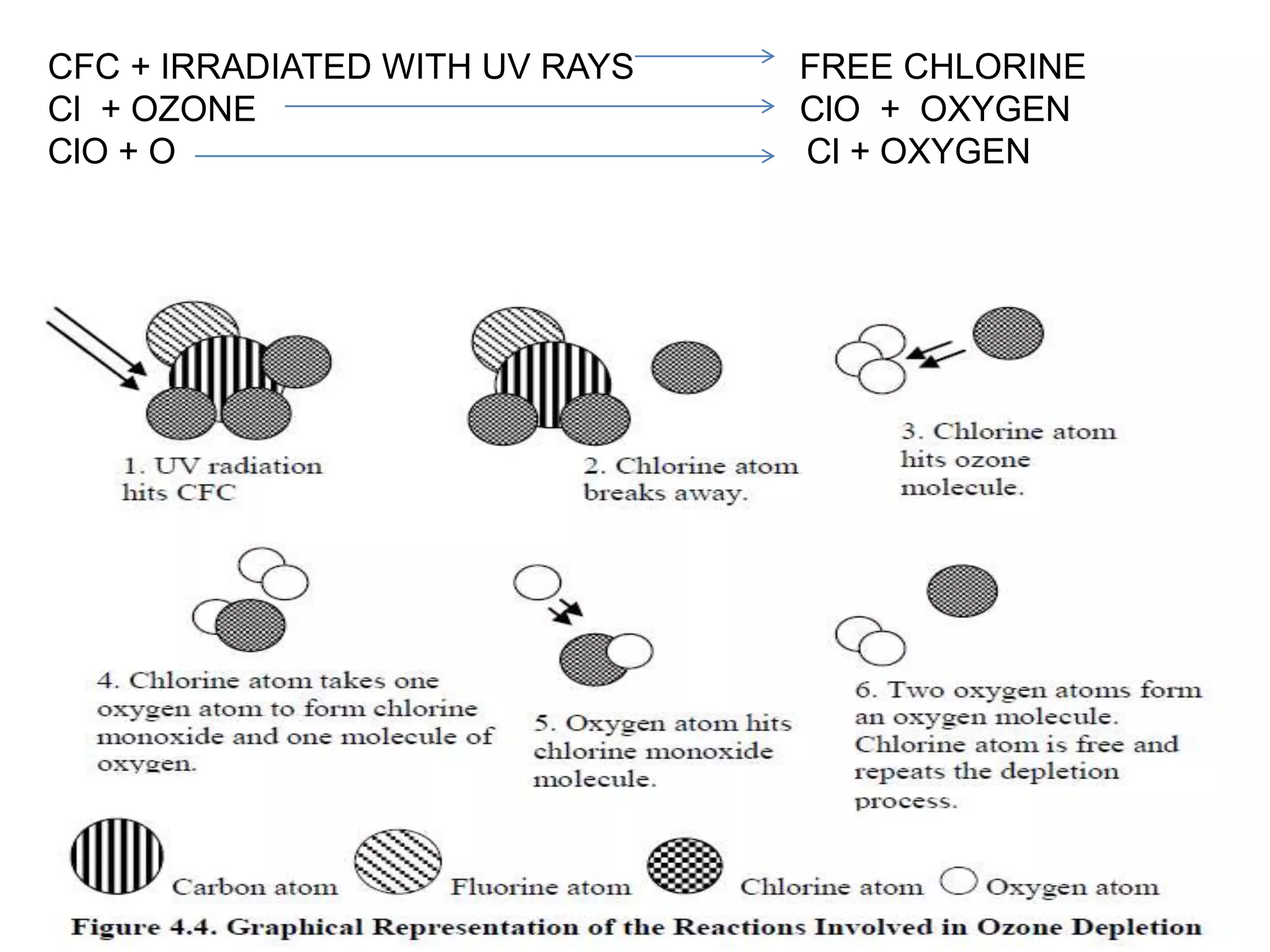
This document provides information on refrigerants including their definition, history, classification, properties, and environmental impact. It discusses early natural refrigerants and the development of artificial refrigerants over time. Refrigerants are classified based on their working principle and chemical properties. Key criteria for refrigerant selection include thermodynamic properties, environmental and safety factors, and cost. Common synthetic refrigerants discussed are CFCs, HCFCs, HFCs, hydrocarbons, and inorganic refrigerants like ammonia and carbon dioxide.