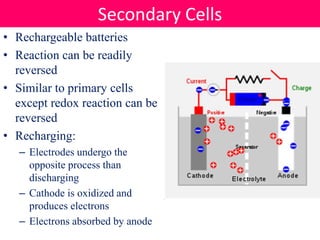
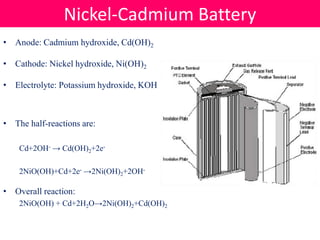
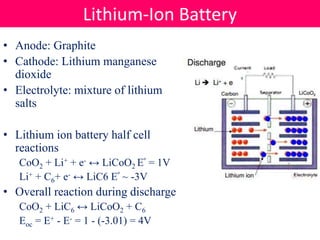
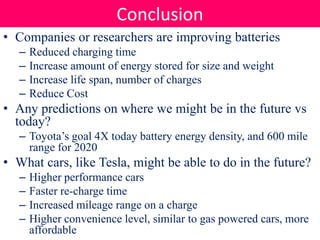
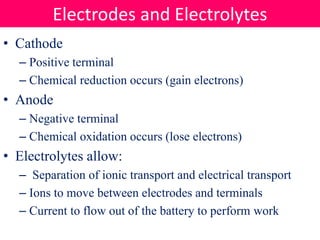
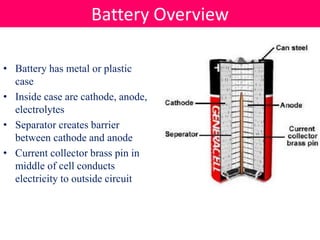
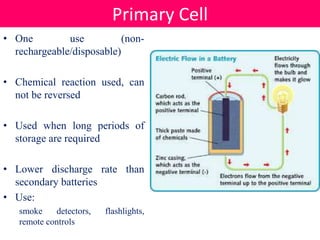
Batteries convert stored chemical energy into electrical energy through electrochemical reactions between electrodes and electrolytes. There are primary batteries that cannot be recharged and secondary batteries that can be recharged. Common battery types include alkaline batteries using zinc and manganese dioxide electrodes, zinc-carbon batteries using zinc electrodes and acidic electrolytes, nickel-cadmium batteries, lead-acid batteries, and lithium-ion batteries widely used in electronics. New battery technologies aim to increase energy density, lifespan, and reduce costs and charging times.



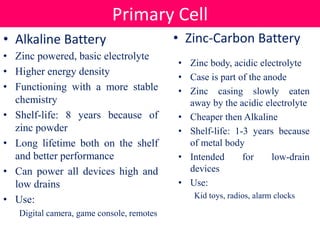
![• Alkaline batteries name came from the electrolyte in an alkane
• Anode: zinc powder form
• Cathode: manganese dioxide
• Electrolyte: potassium hydroxide
• The half-reactions are:
Zn(s) + 2OH−
(aq) → ZnO(s) + H2O(l) + 2e− [e° = -1.28 V]
2MnO2(s) + H2O(l) + 2e− → Mn2O3(s) + 2OH−
(aq) [e° = 0.15 V]
• Overall reaction:
Zn(s) + 2MnO2(s) → ZnO(s) + Mn2O3(s) [e° = 1.43 V]
Alkaline Battery](https://image.slidesharecdn.com/batteries-170720051805/85/Batteries-7-320.jpg)
![• Anode: zinc metal body (Zn)
• Cathode: manganese dioxide (MnO2)
• Electrolyte: paste of zinc chloride and ammonium chloride dissolved in water
• The half-reactions are:
Zn(s) → Zn2+(aq) + 2e- [e° = -0.763 V]
2NH4
+(aq) + 2MnO2(s) + 2e- → Mn2O3(s) + H2O(l) + 2NH3(aq) + 2Cl- [e° = 0.50 V]
• Overall reaction:
Zn(s) + 2MnO2(s) + 2NH4Cl(aq) → Mn2O3(s) + Zn(NH3)2Cl2 (aq) + H2O(l) [e° = 1.3 V]a
Zinc-Carbon Battery](https://image.slidesharecdn.com/batteries-170720051805/85/Batteries-8-320.jpg)