This document discusses various battery technologies including primary and secondary cells. It provides details on dry cells, lead-acid batteries, nickel-cadmium batteries, and fuel cells. The key points are:
- Primary cells cannot be recharged while secondary cells can be recharged by passing current in the opposite direction.
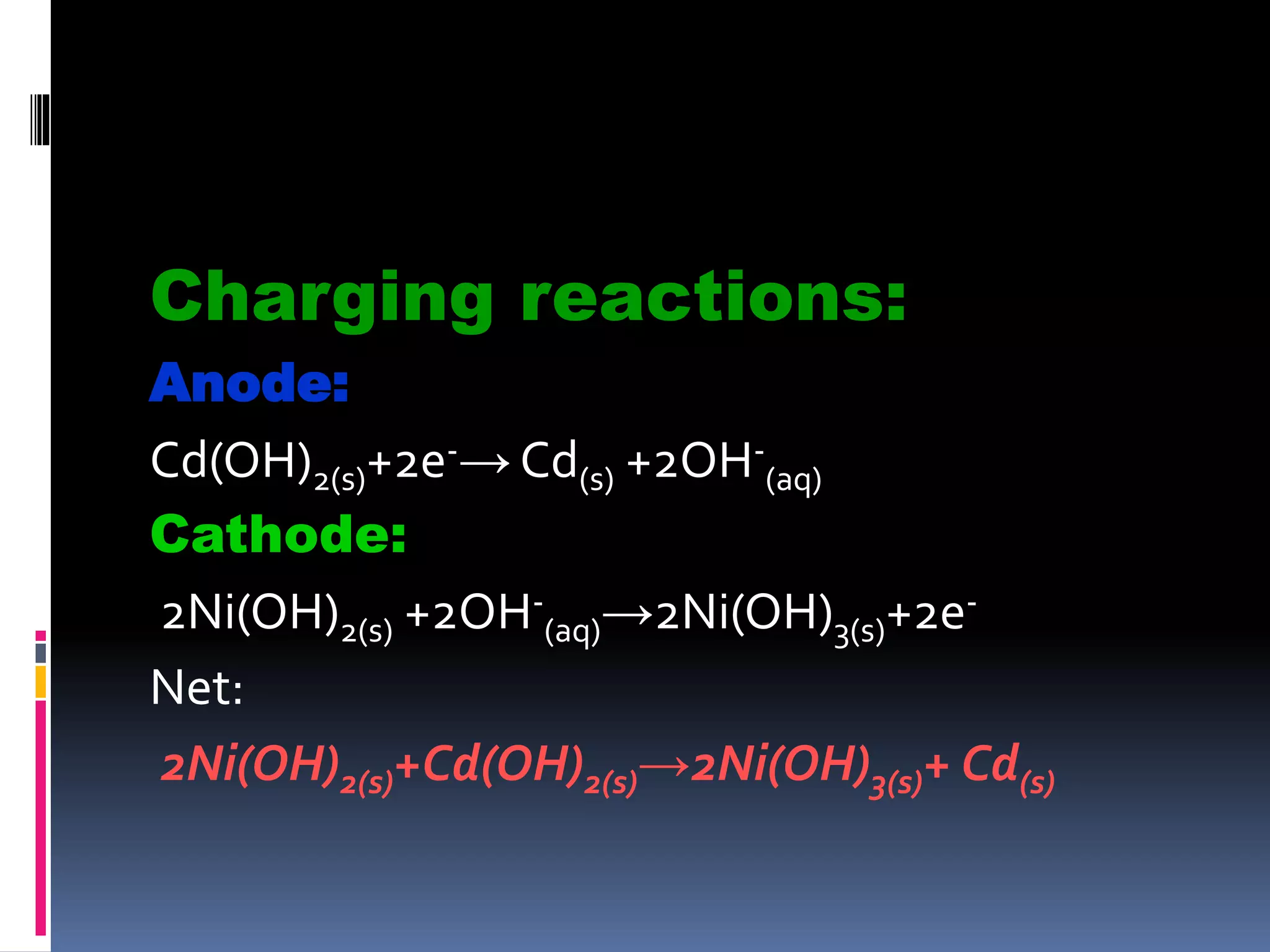
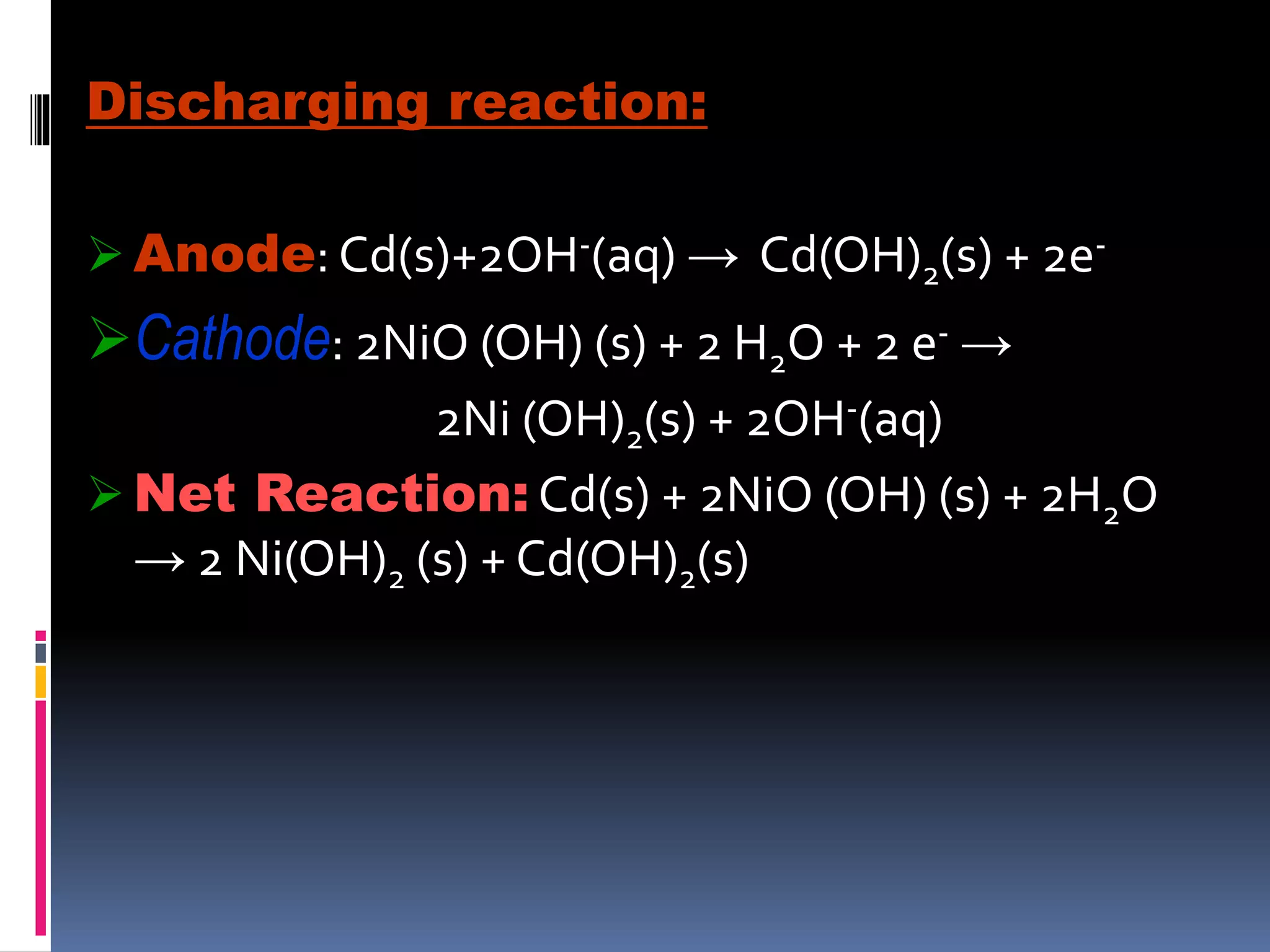
- Dry cells are inexpensive but have a limited shelf life. Lead-acid batteries are rechargeable and commonly used in vehicles. Nickel-cadmium batteries can be recharged hundreds of times.
- Fuel cells directly convert chemical energy to electrical energy and include hydrogen-oxygen and methanol-oxygen types. They do not require recharging and have applications in space, military, and stationary power









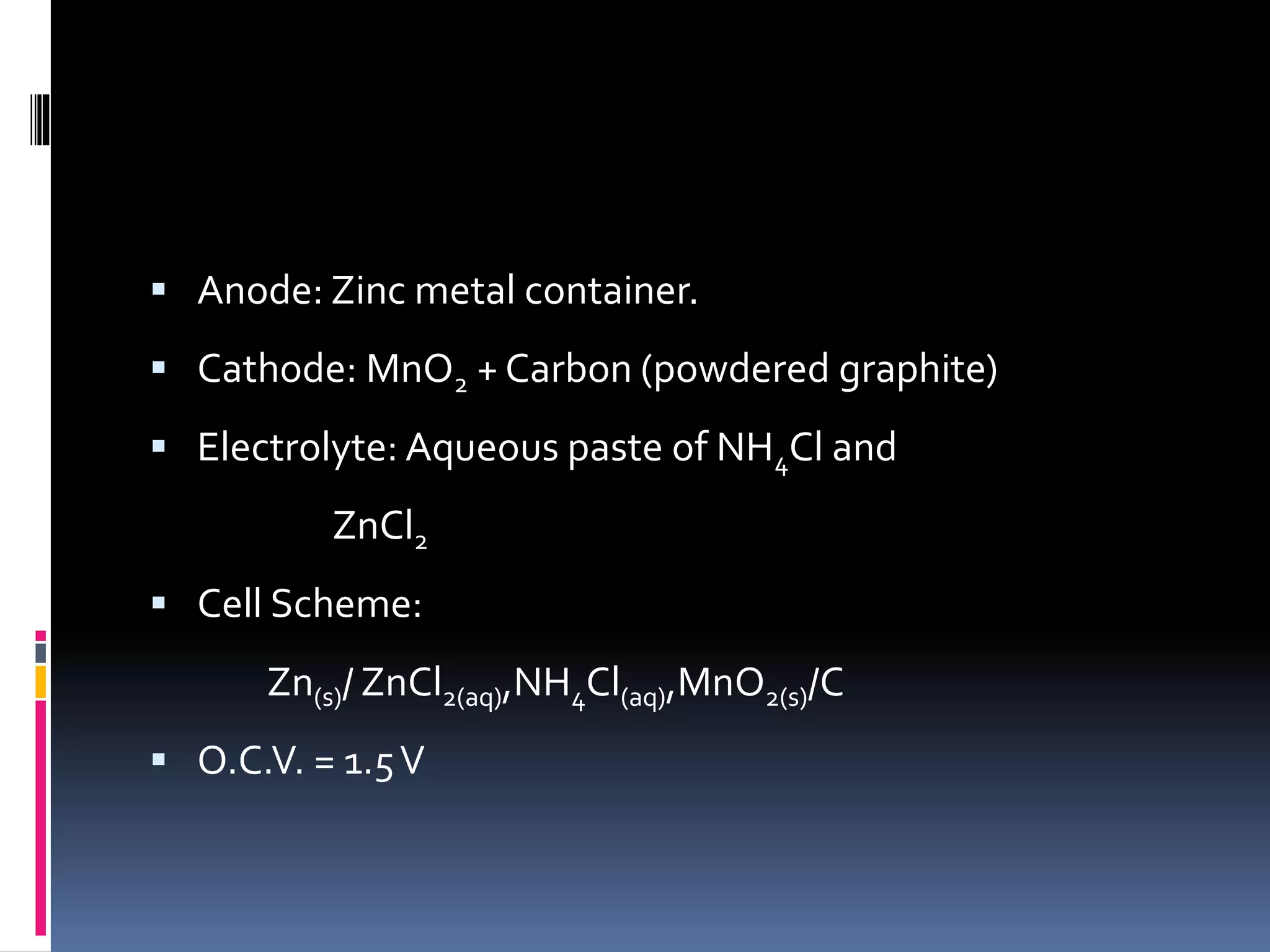

![Secondary Reactions:
NH4
+
(aq)+OH-
(aq) → NH3(g)+H2O(l)
Zn2+
(aq)+2NH3(s)+2Cl- → [Zn(NH3)2 Cl2]
Zn + 2MnO2 + 2NH4Cl →[Zn(NH3)2Cl2]+ H2O+
Mn2O3](https://image.slidesharecdn.com/batterytechnology-160416165418/75/Battery-Types-and-Battery-technology-12-2048.jpg)