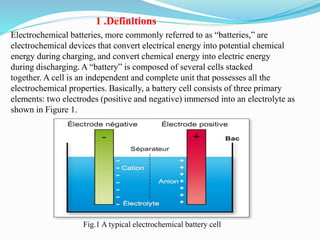
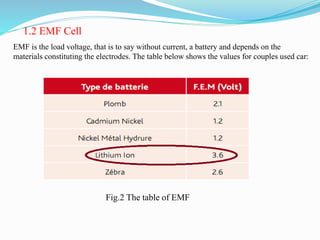
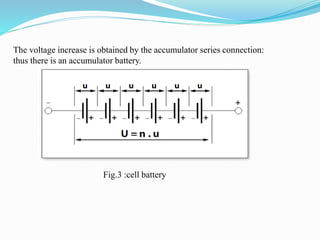
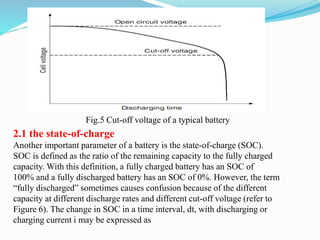
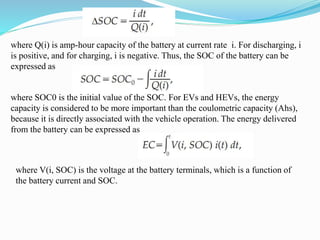
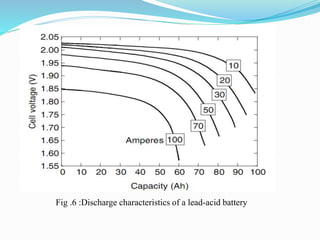
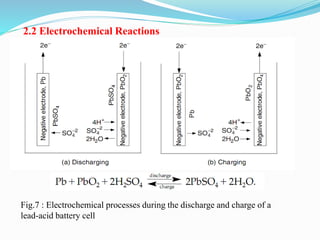
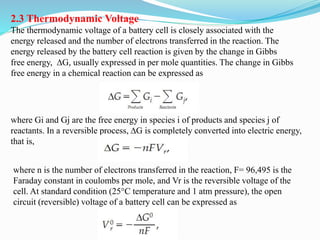
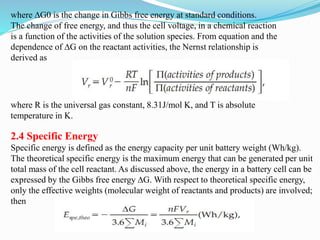
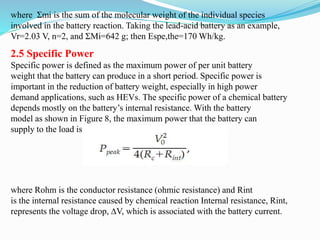
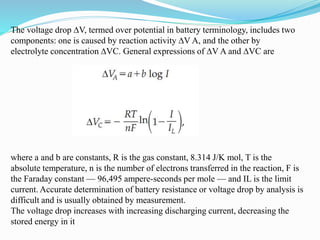
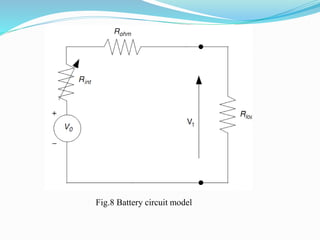
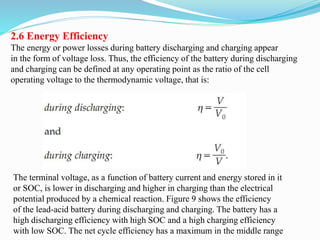
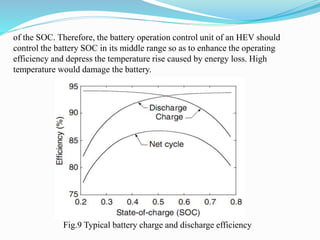
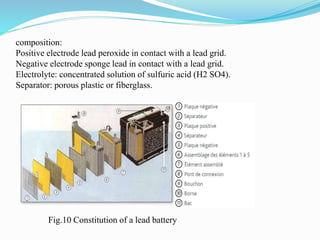
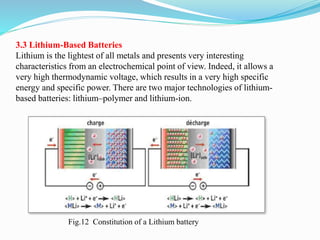
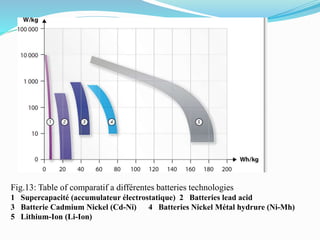
The document discusses energy storage systems and their applications in electric vehicles. It provides details on different battery technologies used in HEVs, including their composition, characteristics, and parameters. Lead-acid batteries are currently most widely used due to their low cost but lithium-ion batteries have higher energy density and are gaining popularity. The document compares various battery technologies such as lead-acid, nickel-cadmium, nickel-metal hydride, and lithium-ion in terms of their specifications and suitability for electric vehicles.