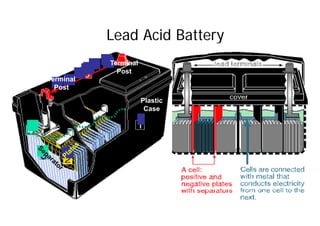
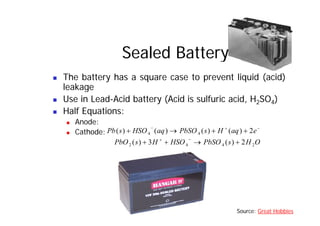
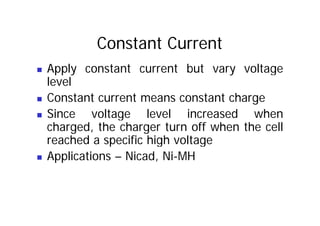
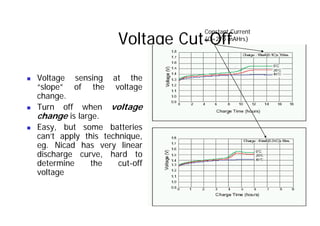
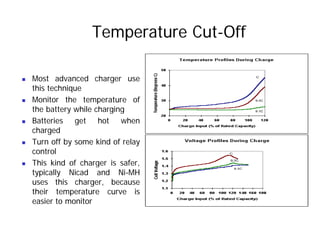
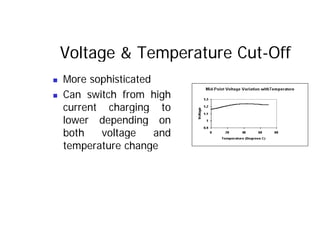
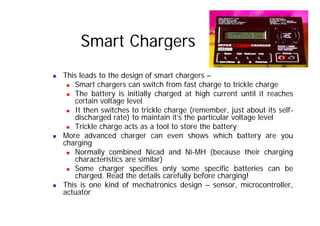
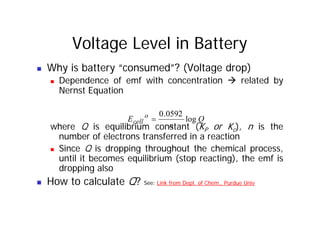
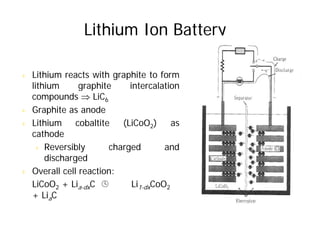
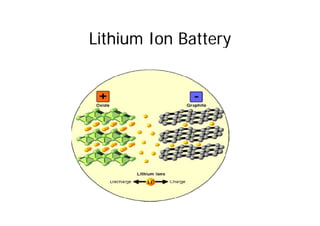
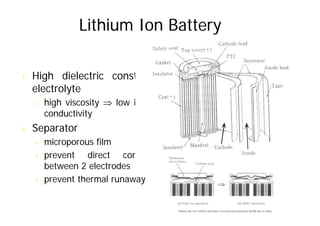
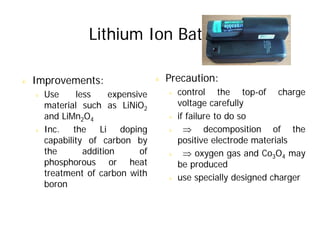
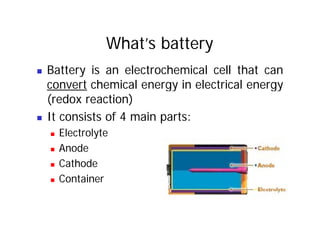
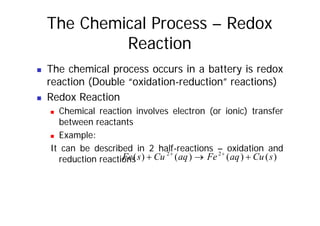
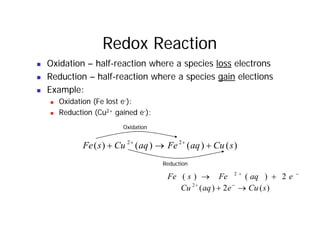
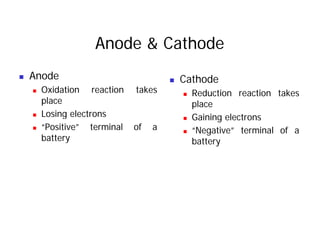
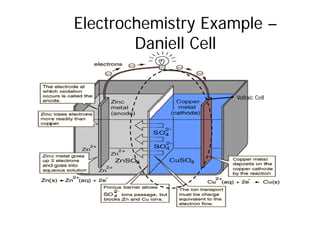
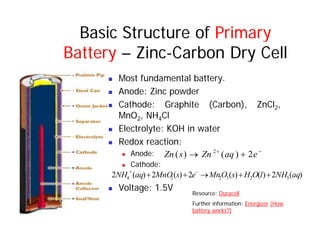
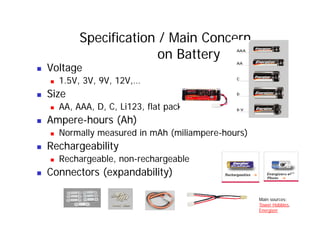
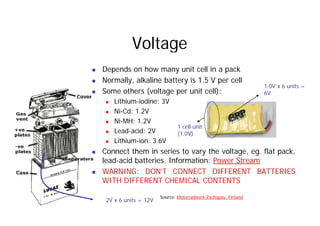
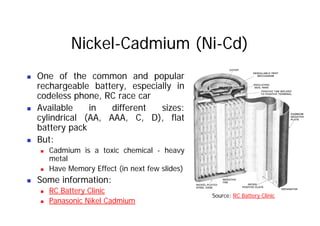
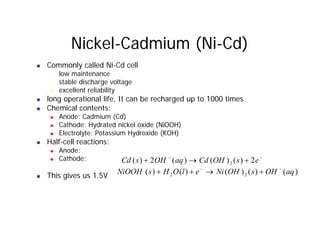
This document provides information about different types of batteries. It begins with an overview of the basic electrochemistry involved in batteries, including the components (electrolyte, anode, cathode, container) and redox reactions. It then discusses various primary (non-rechargeable) batteries like zinc-carbon, silver oxide, and alkaline batteries. Rechargeable batteries covered include nickel-cadmium (Ni-Cd), nickel-metal hydride (Ni-MH), lead-acid, and lithium-ion. Specifications discussed are voltage, size, capacity (amp-hours), and rechargeability. Concerns with different battery types like toxicity (cadmium) and memory effect are also summarized.












![Nickel-Metal Hydride (Ni-MH)
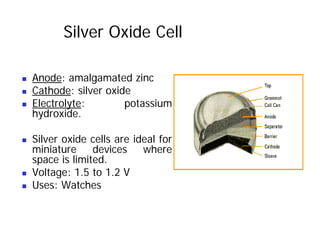
Nickel Metal Hydride (Ni MH)
„ Chemical content:
„ Cathode: Metal Hydrides
„ Cathode: Metal Hydrides
„ Anode: Nickel
„ Electrolyte: Potassium hydroxide
„ Electrolyte: Potassium hydroxide
(KOH)
„ Half equations:
q
„ Charging:
„ Anode:
−
−
+
→
+
+ OH
H
Alloy
e
O
H
Alloy ]
[
2
„ Cathode:
„ Discharging
Anode:
−
−
+
+
→
+ e
O
H
NiOOH
OH
OH
Ni 2
2
)
(
−
−
O
H
All
OH
H
All ]
[
Source:
Extreme Tech
„ Anode:
„ Cathode:
+
+
→
+ e
O
H
Alloy
OH
H
Alloy 2
]
[
−
−
+
→
+
+ OH
OH
Ni
e
O
H
NiOOH 2
2 )
(](https://image.slidesharecdn.com/batterytechnology-220918175440-a52d7ca7/85/BatteryTechnology-pdf-26-320.jpg)