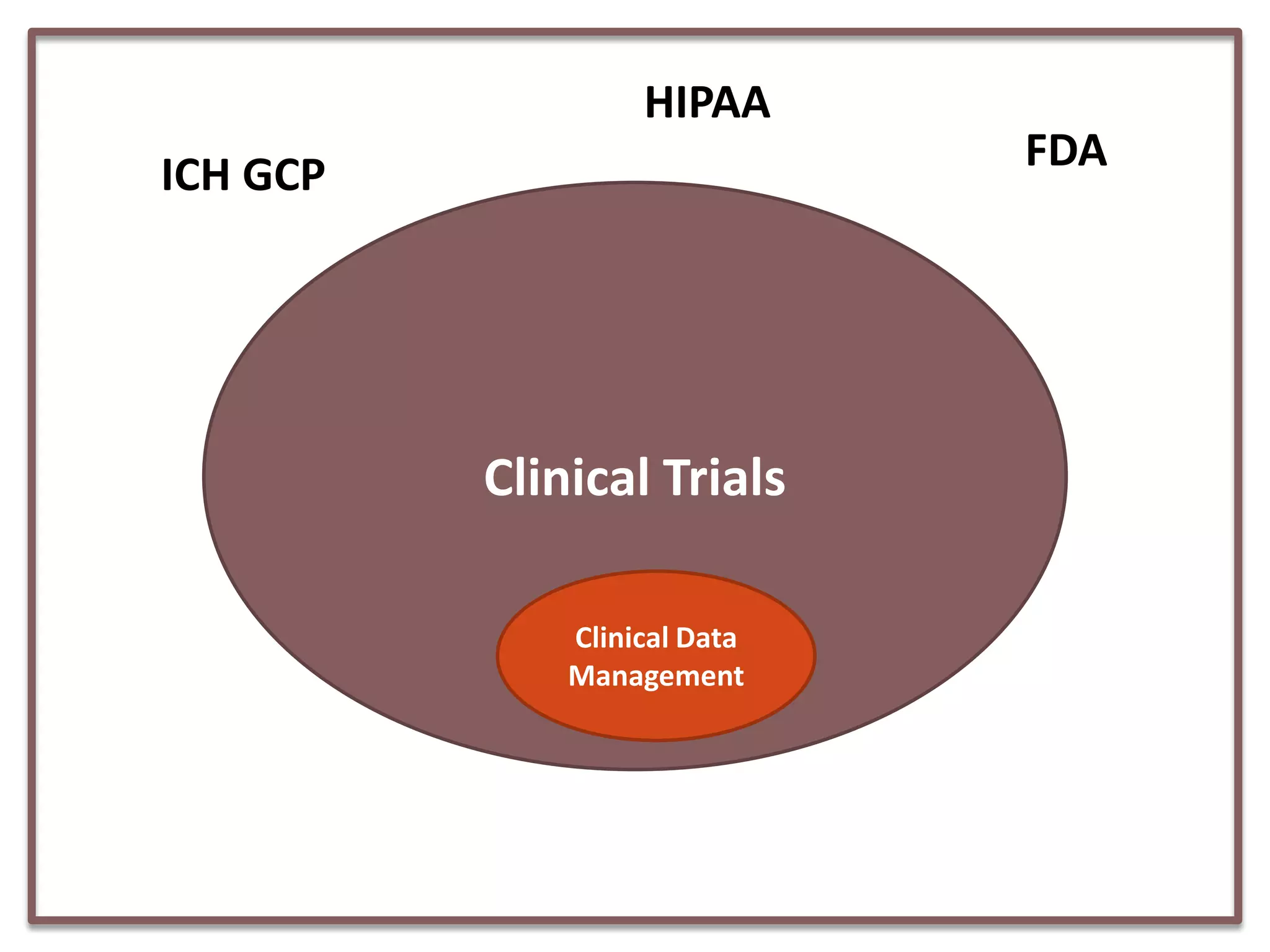
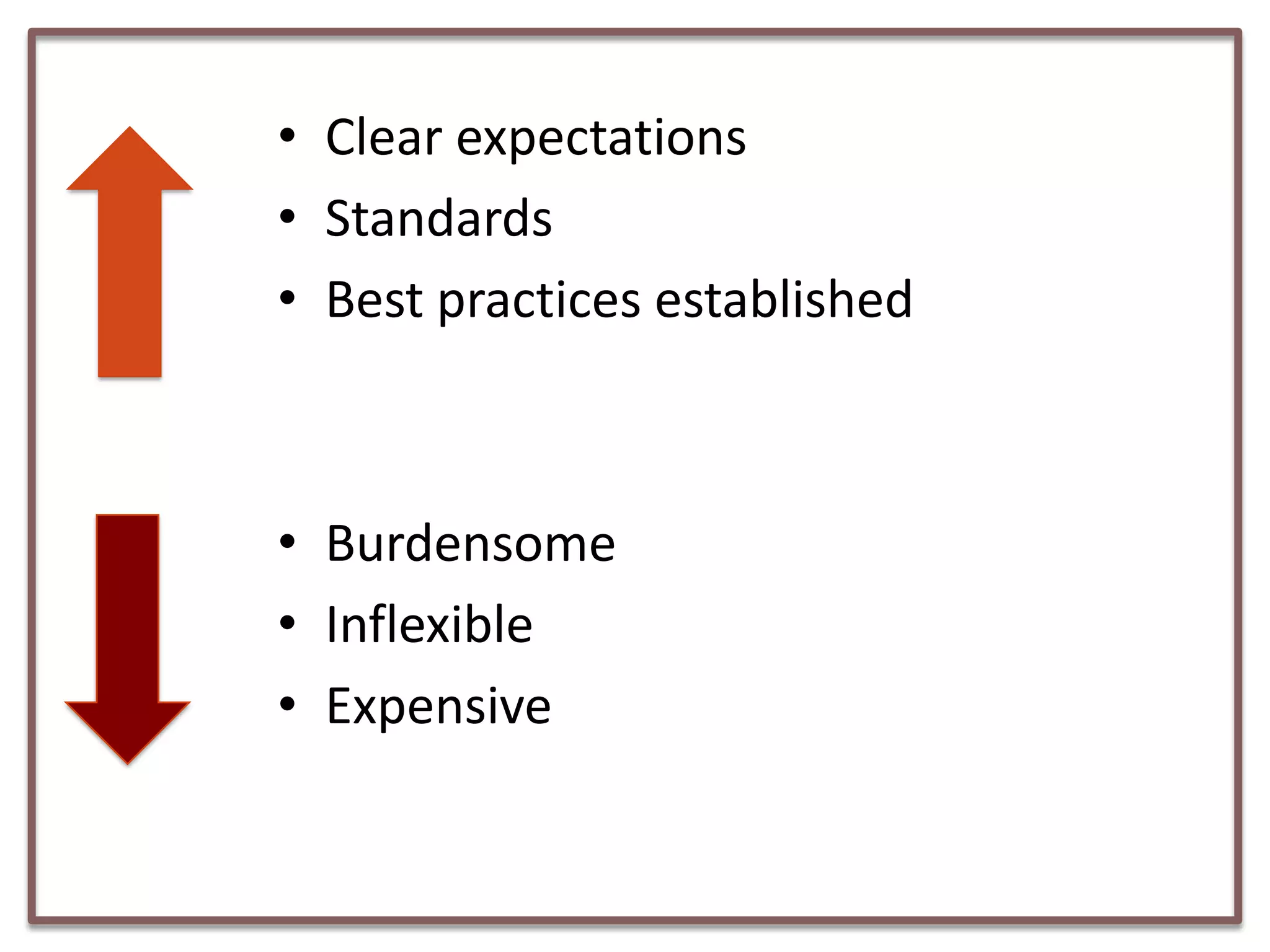
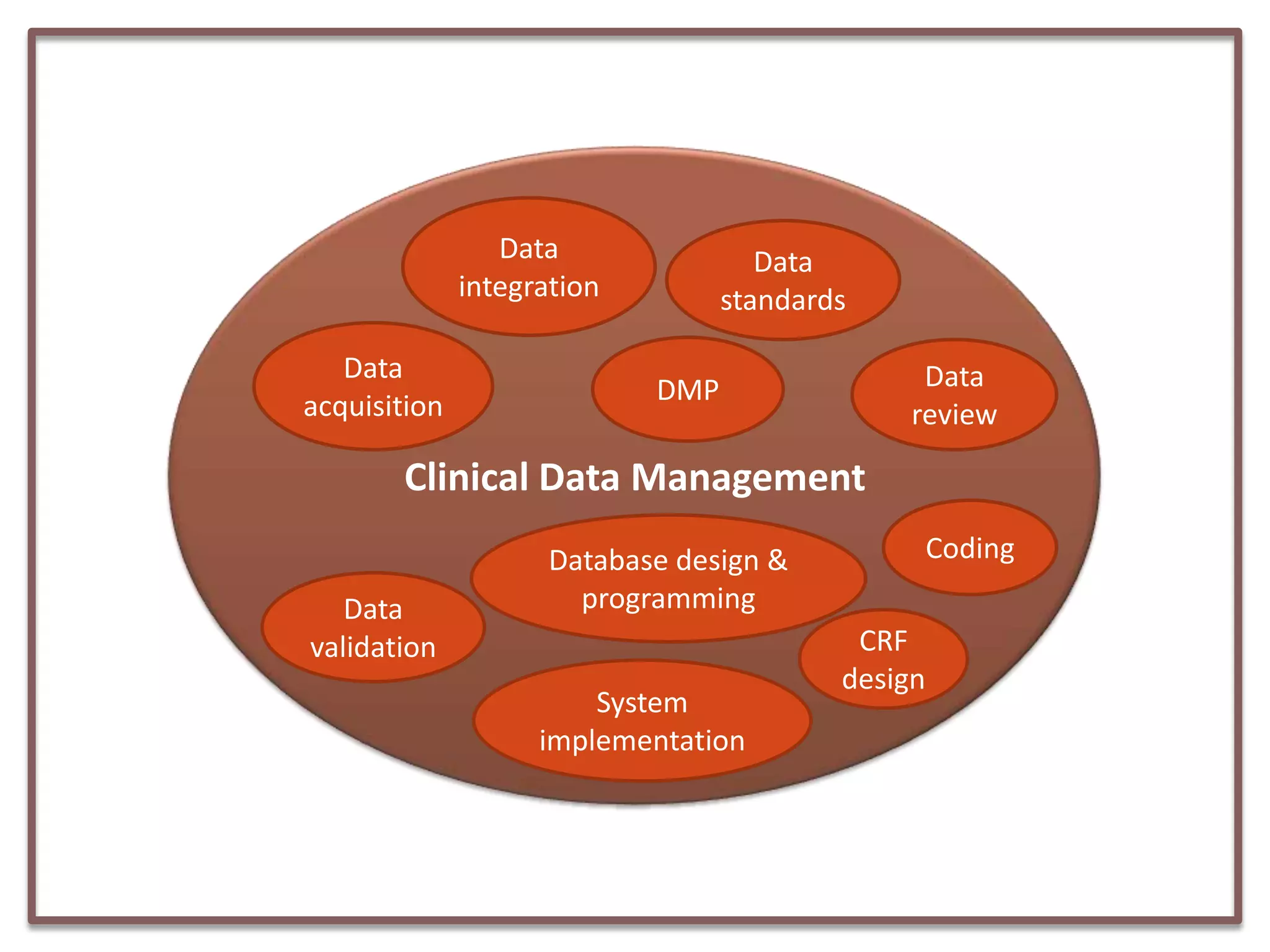
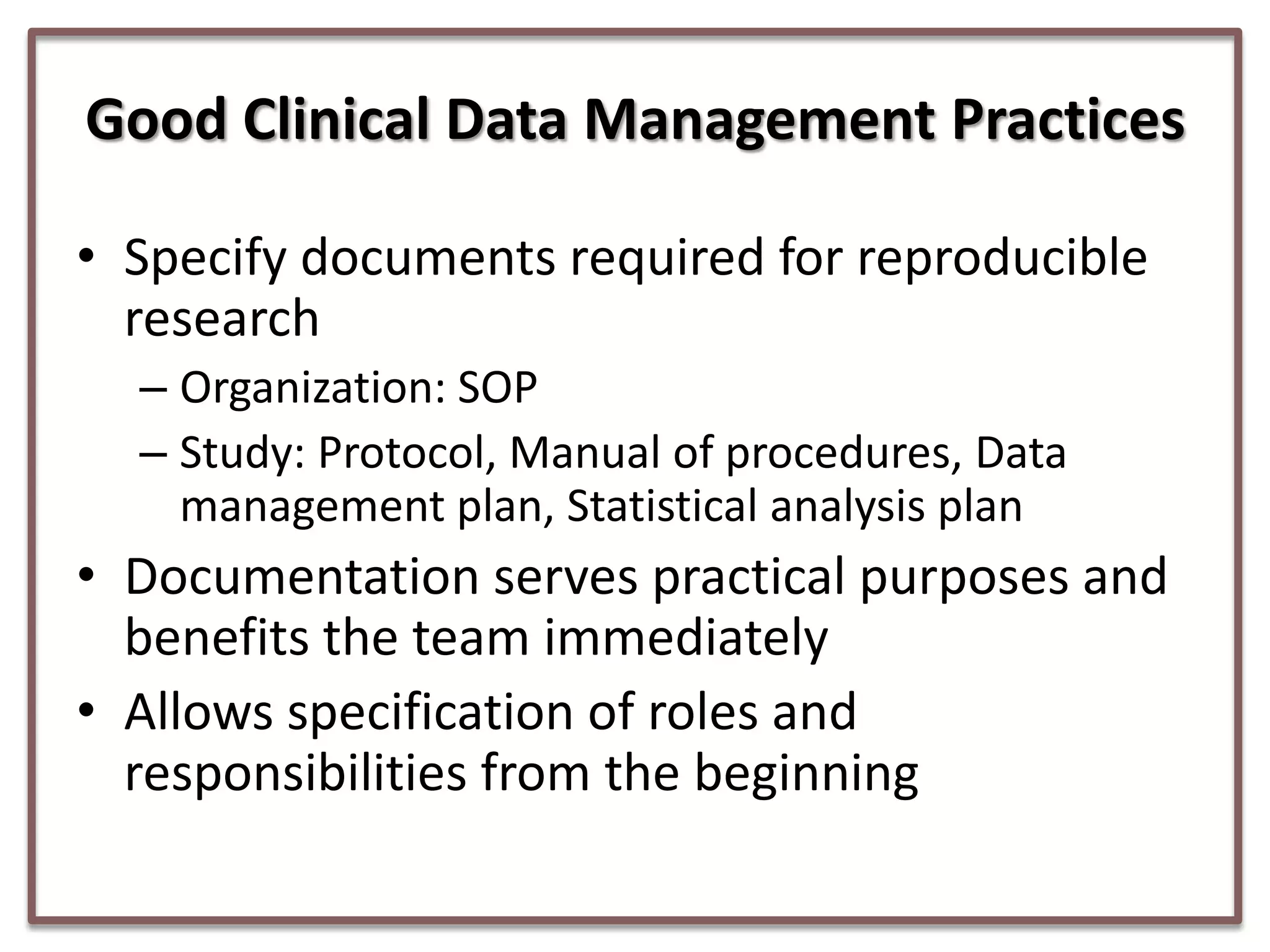
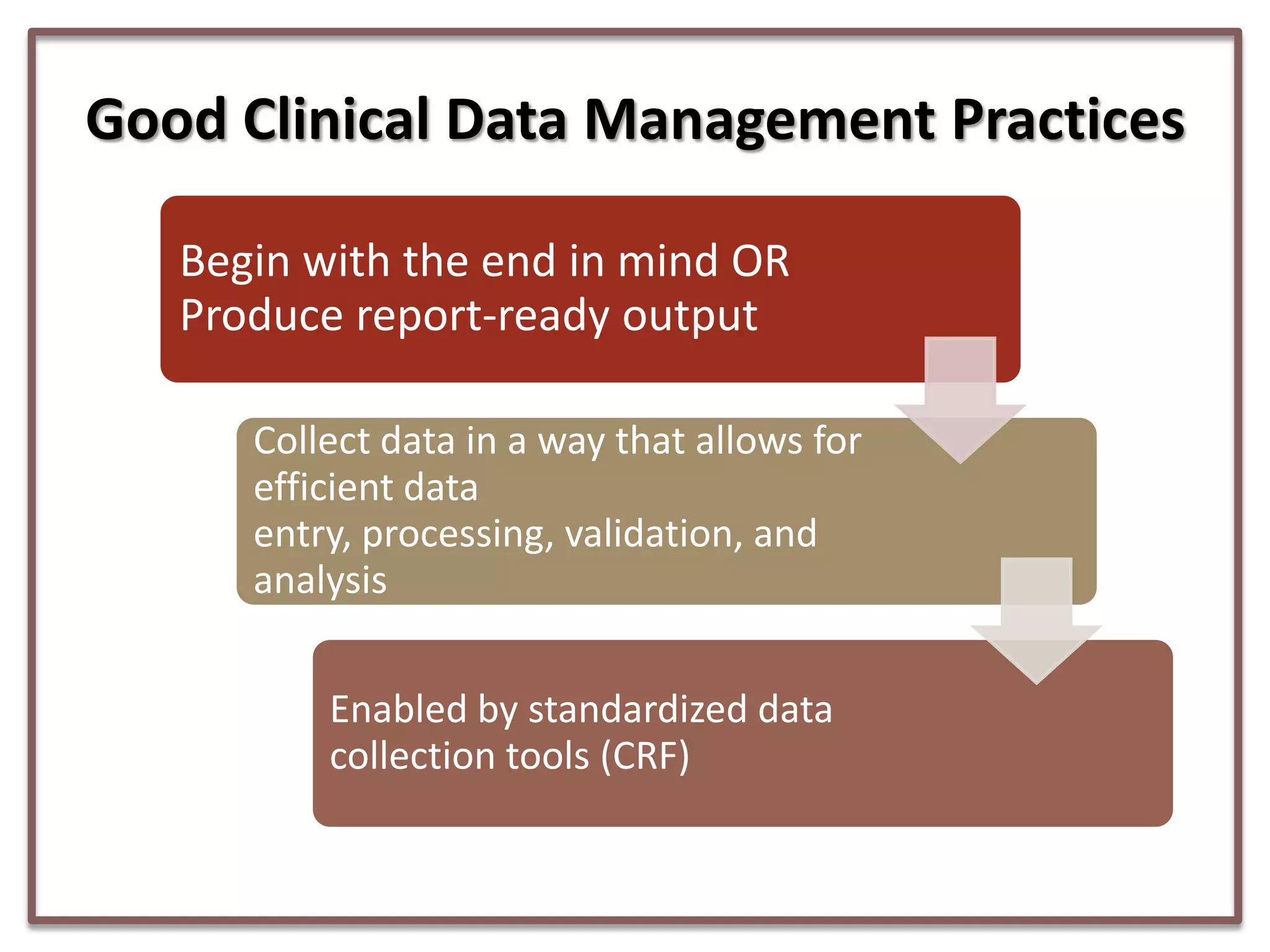
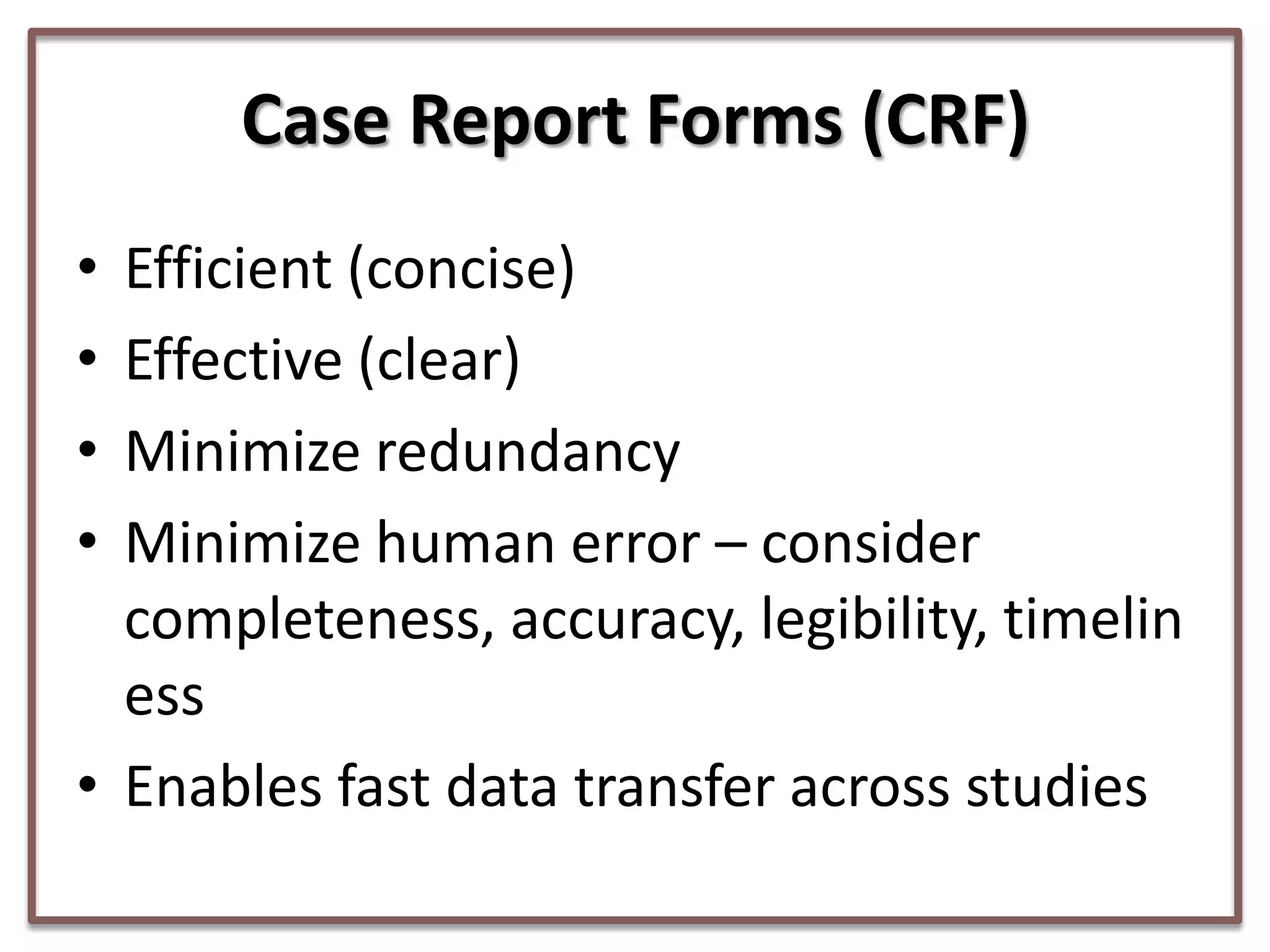
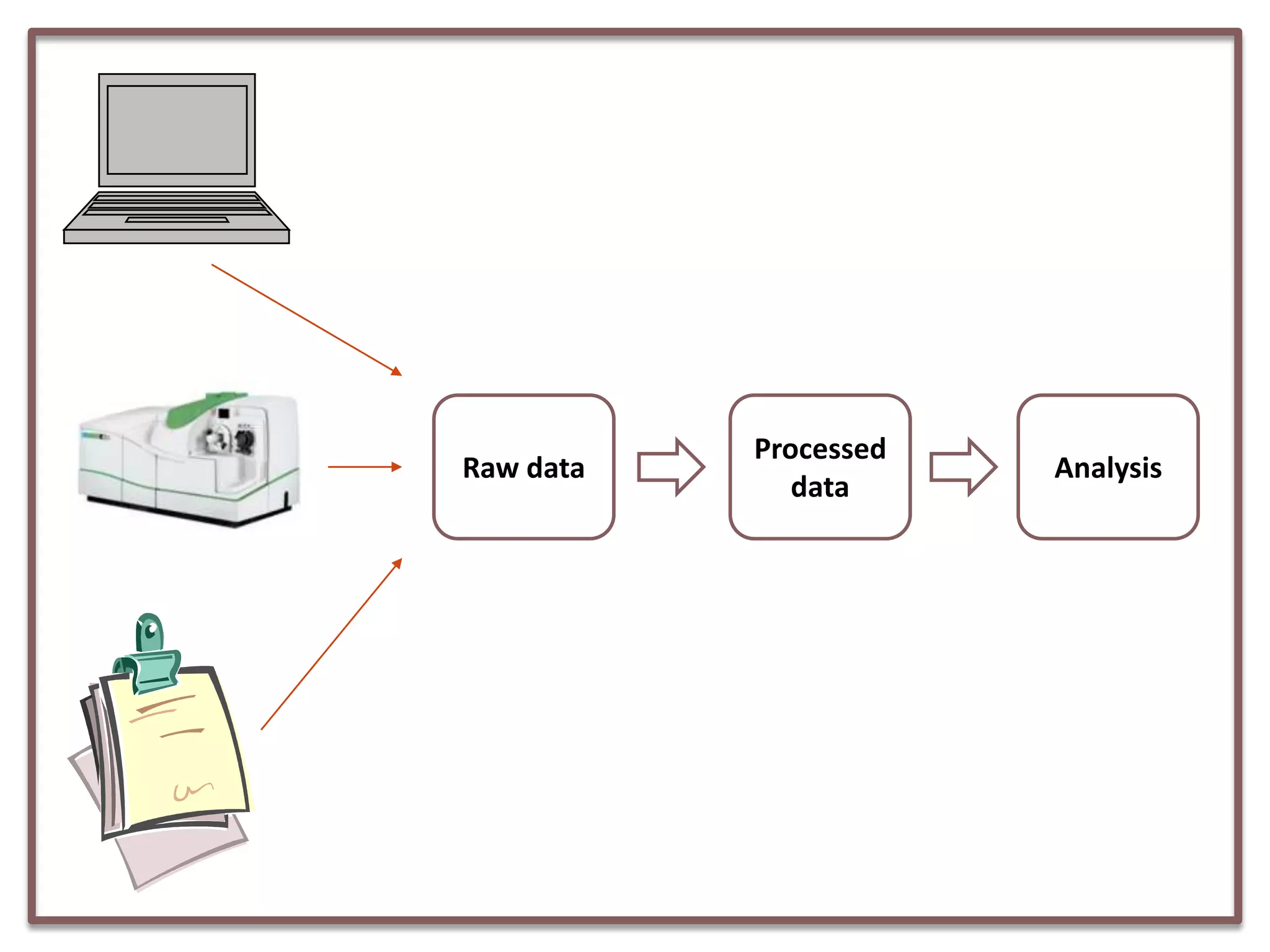
This document summarizes a presentation on clinical data management strategies for unregulated data. It discusses how regulations like HIPAA and ICH GCP have established standard practices for regulated clinical trials in areas like efficiency, safety, accuracy, and privacy. However, these standards can be burdensome and inflexible for unregulated data. The presentation therefore outlines good clinical data management practices from a 2011 document that focus on planning, documentation, stakeholder involvement, and producing report-ready output to enable efficient data collection, processing, and analysis. It emphasizes beginning with the end in mind and using standardized case report forms and data collection tools.