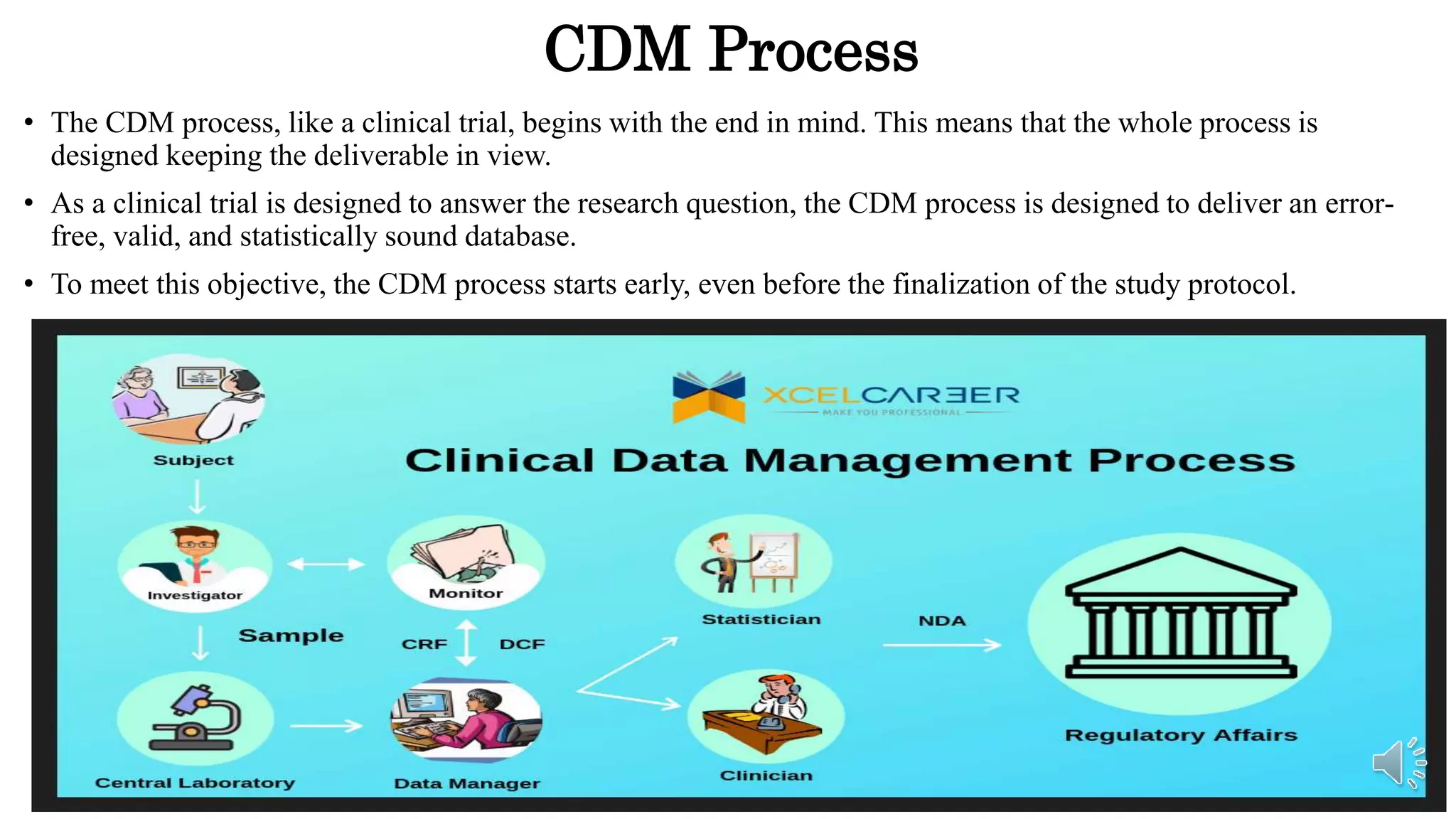
Clinical Data Management (CDM) is vital in clinical trials for ensuring the collection and management of quality data that adheres to regulatory standards. It involves multiple activities and professionals to process data effectively, ultimately aiming to support ethical drug development and patient health. This document outlines the importance of CDM, activities involved, relevant regulations, and tools used in the field.

![What is Clinical Data Management [CDM] ?
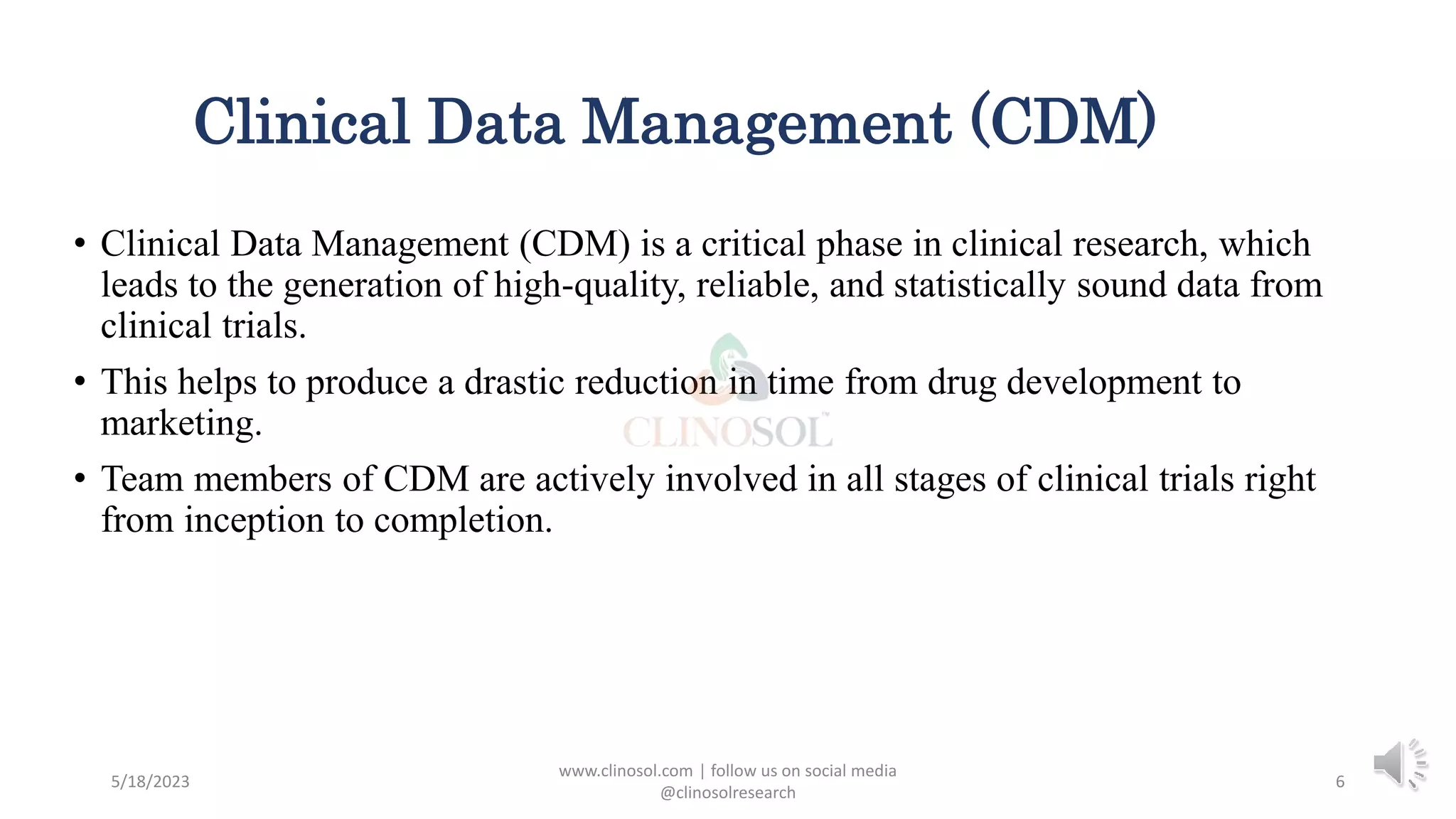
• Clinical data management (CDM) is the process of collecting and managing research data
in accordance with regulatory standards to obtain quality information that is complete and
error-free.
• The goal is to gather as much such data for analysis as possible that adheres to federal,
state, and local regulations.
5/18/2023
www.clinosol.com | follow us on social media
@clinosolresearch
2](https://image.slidesharecdn.com/ramyapptupdated-230518130643-9f6edd0d/75/Clinical-Data-Management-importance-in-Clinical-Trials-2-2048.jpg)
![CLINICAL DATA MANAGEMENT [CDM]
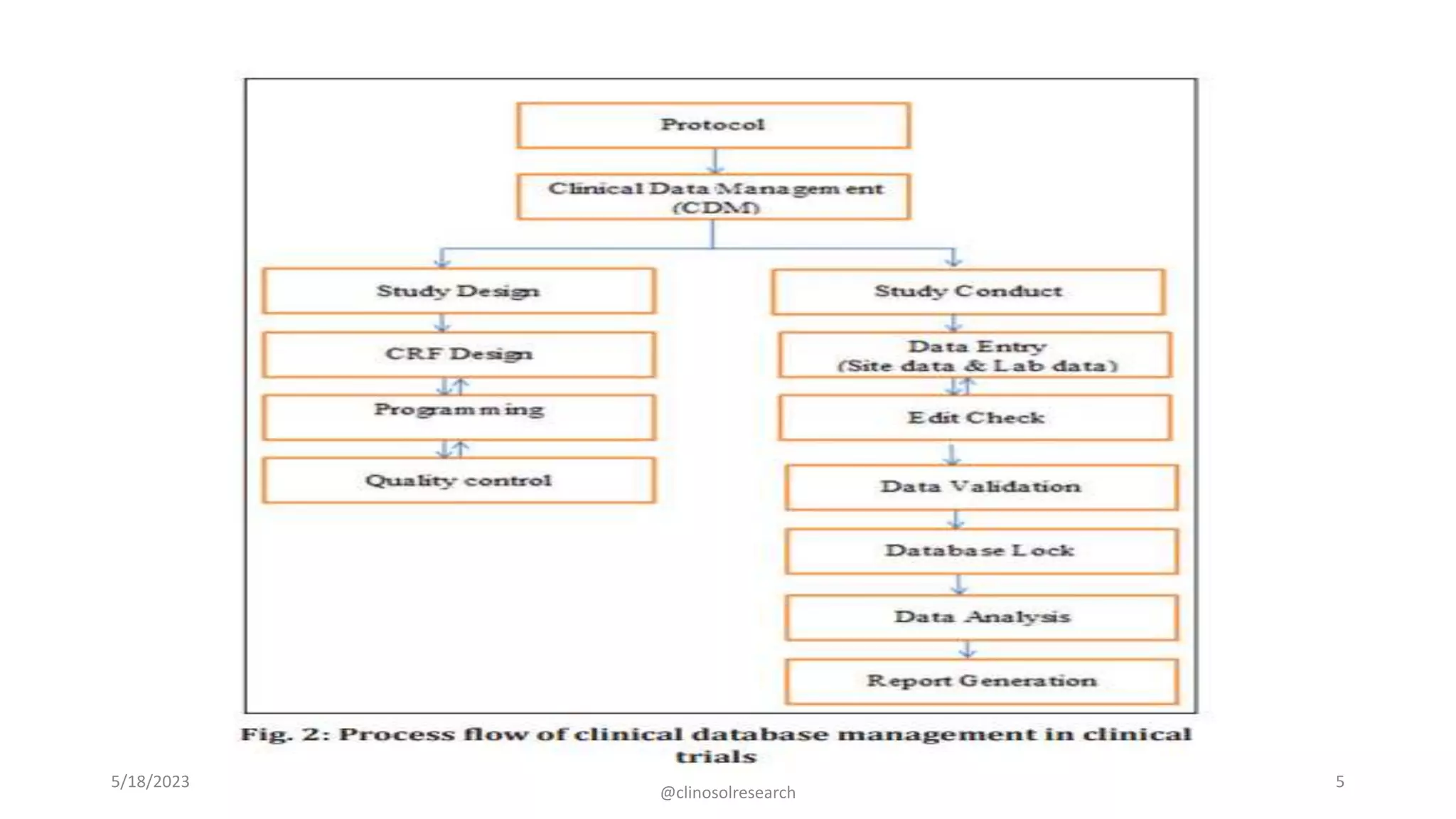
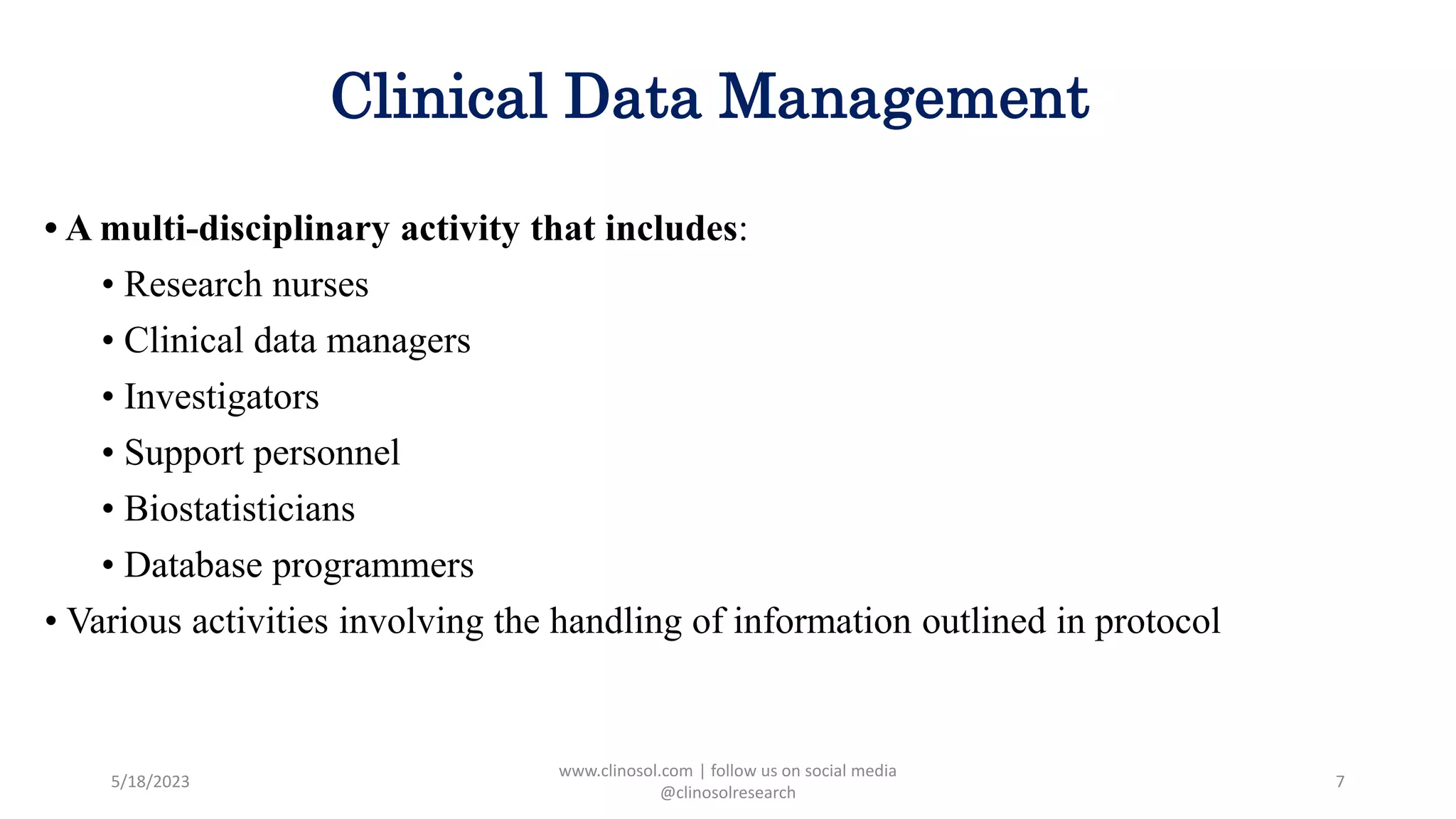
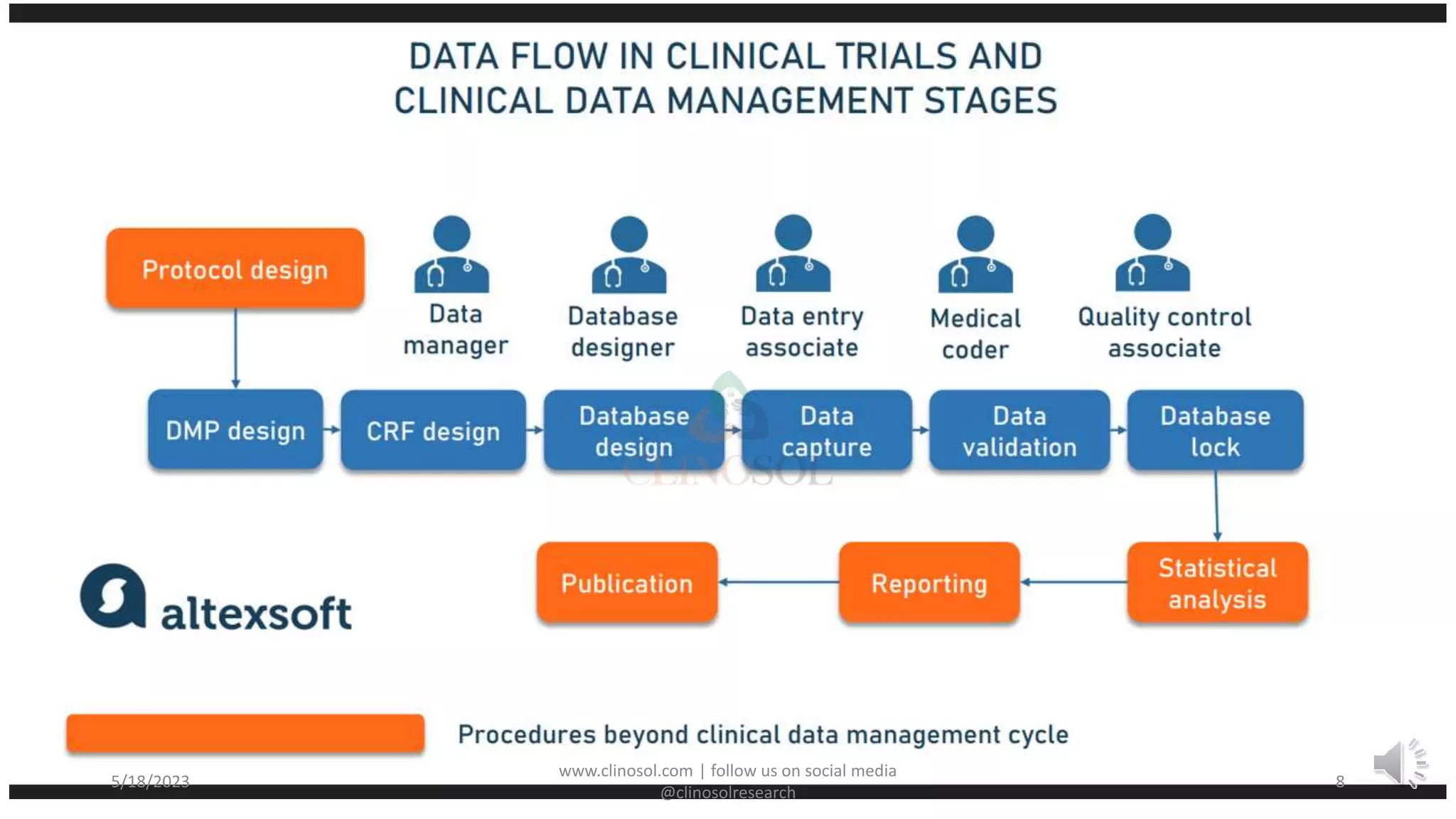
• Clinical data management (CDM) consists of various activities involving the handling of
data or information that is outlined in the protocol to be collected/analyzed. CDM is a
multidisciplinary activity
• Drug developers should ensure that the data submitted to regulatory bodies is definitive
from an ethical point of view.
• It helps to make efficient treatment decisions and ultimately affects patient health.
• For this, the quality of clinical trial data is crucial.
• To achieve this quality data, researchers need to have proper data management in clinical
trials using clinical data management platforms
5/18/2023
www.clinosol.com | follow us on social media
@clinosolresearch
3](https://image.slidesharecdn.com/ramyapptupdated-230518130643-9f6edd0d/75/Clinical-Data-Management-importance-in-Clinical-Trials-3-2048.jpg)