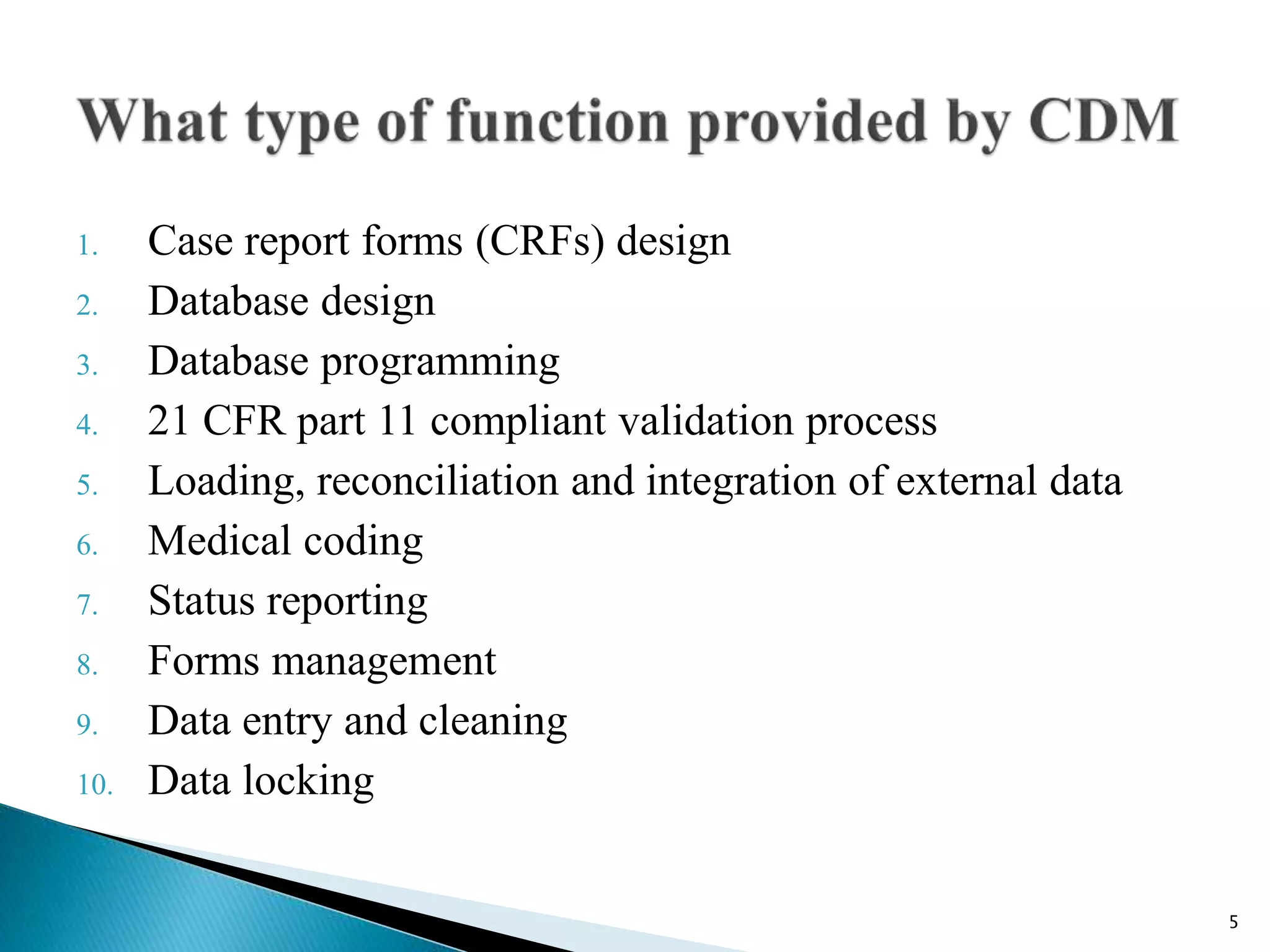
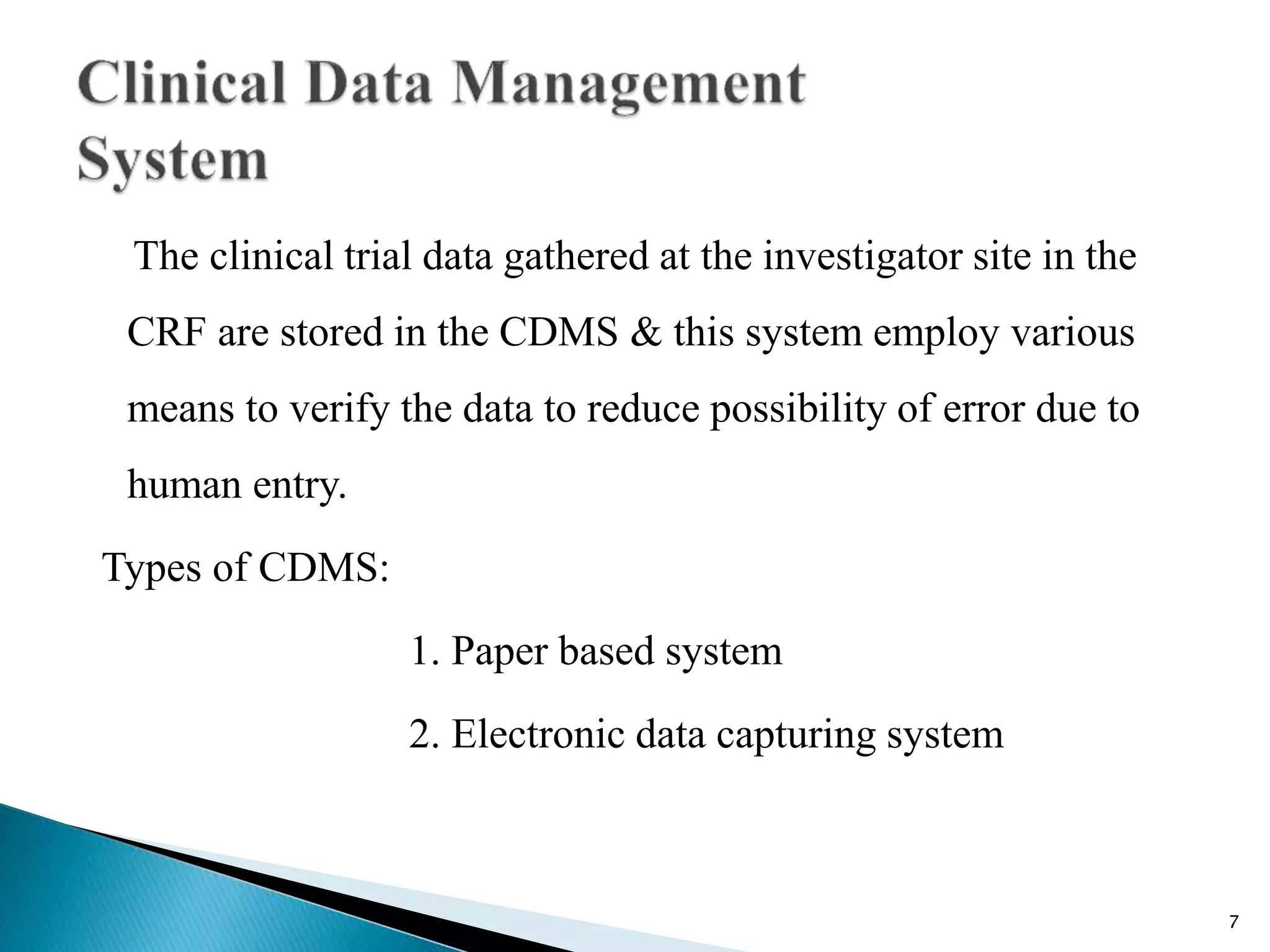
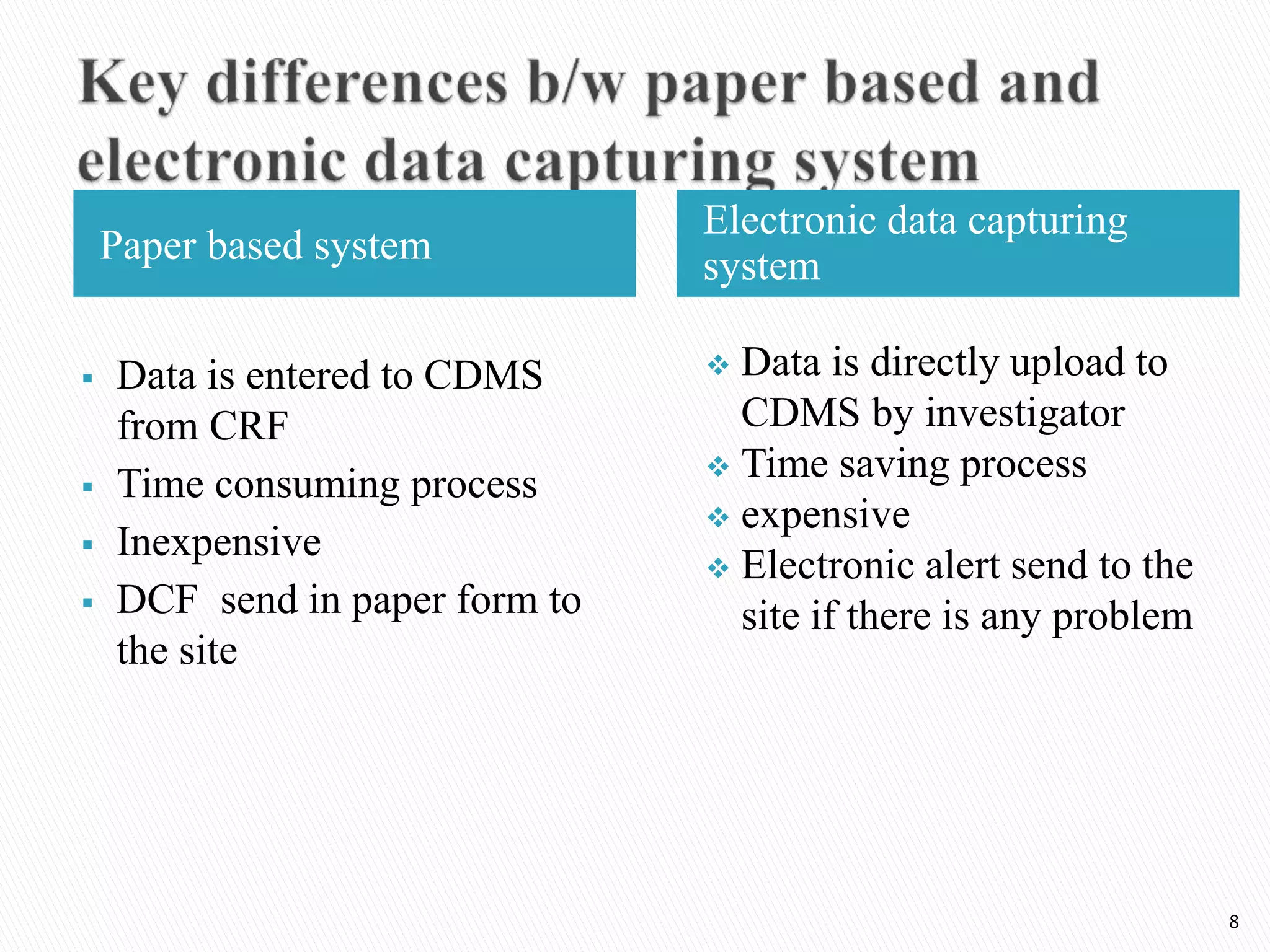
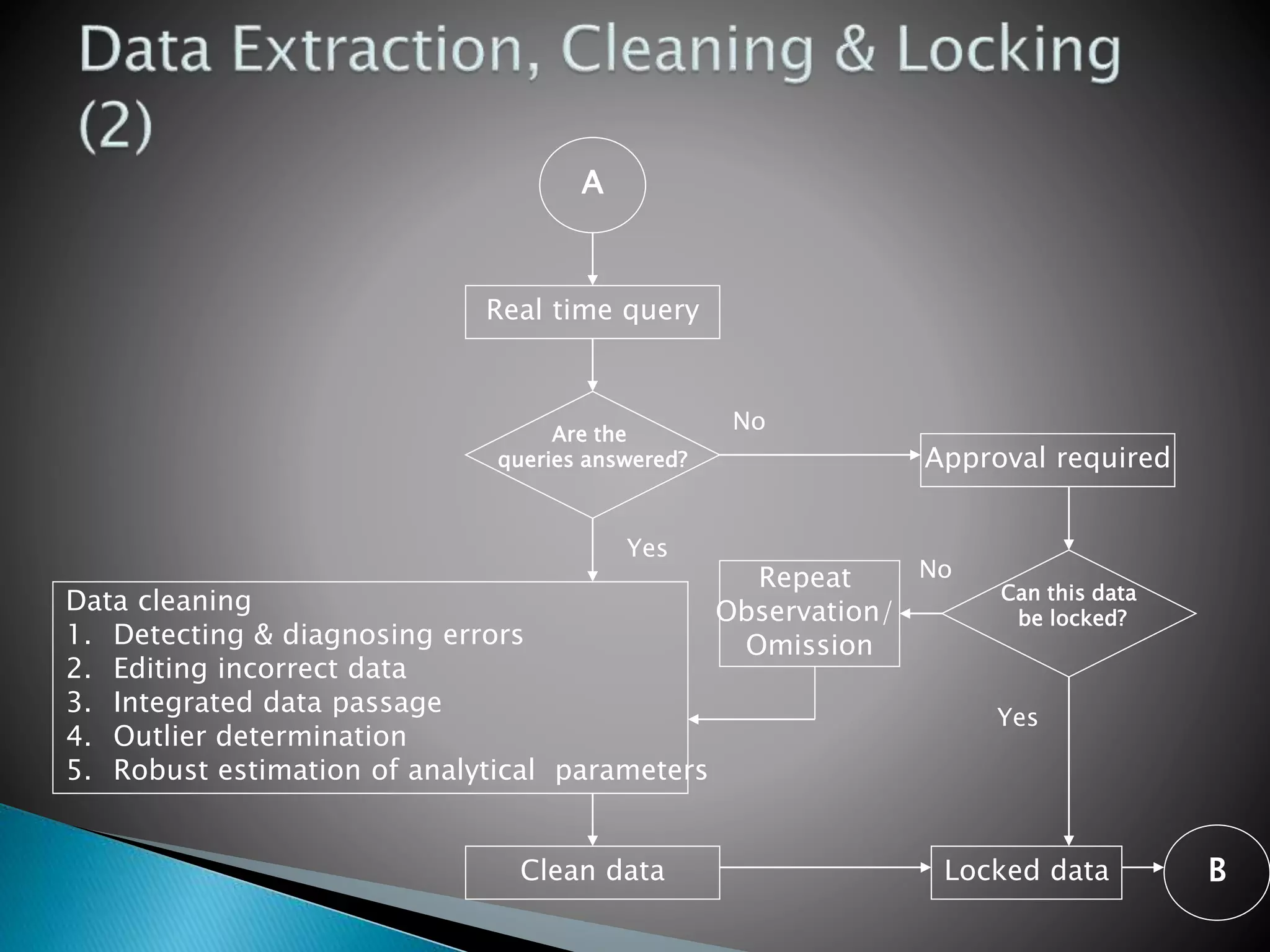
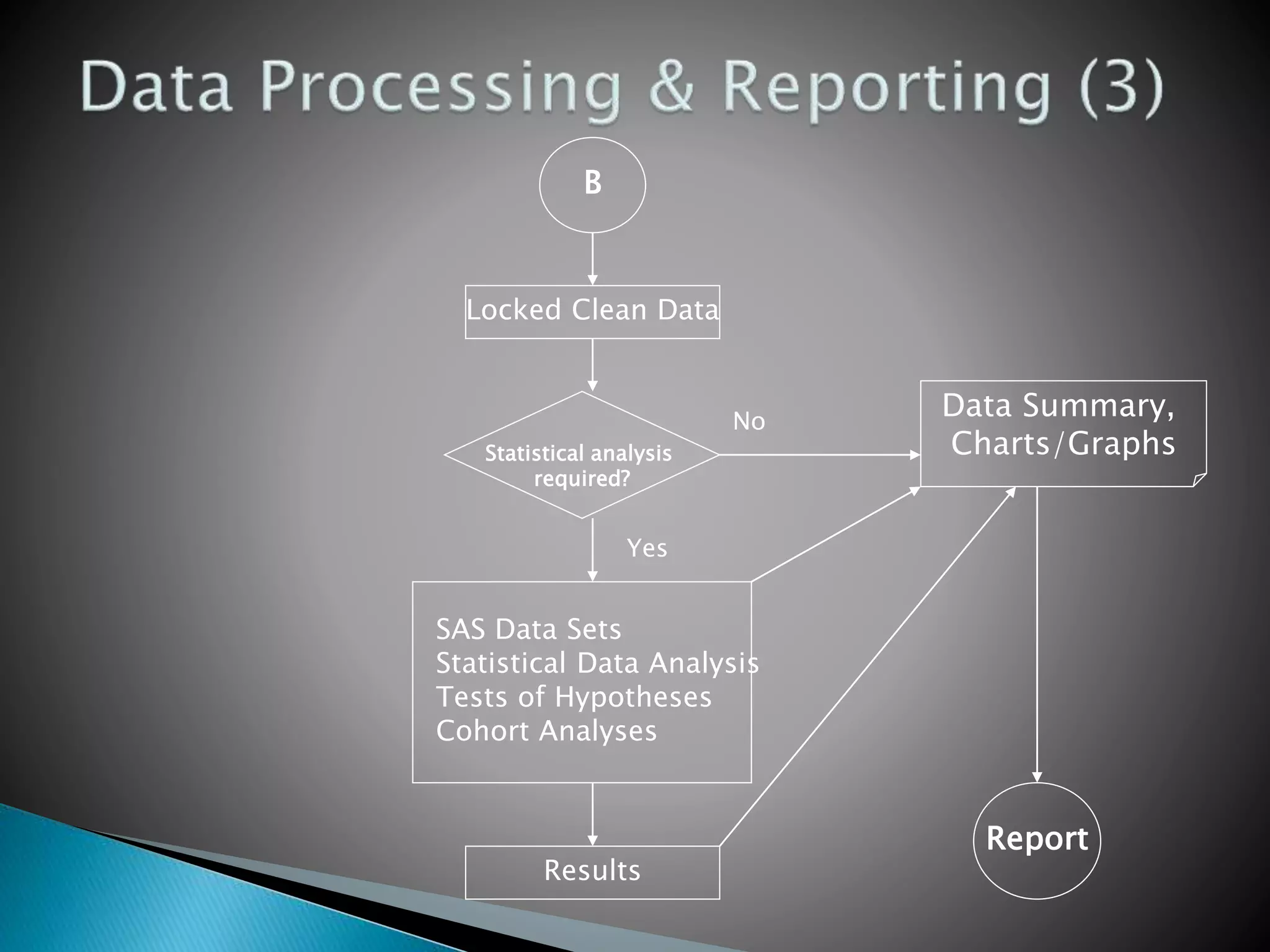
Clinical Data Management (CDM) is a critical phase in clinical research that leads to generating high-quality, reliable data from clinical trials. CDM involves collecting, integrating, and ensuring the availability of appropriate quality and cost data. It encompasses entering, verifying, validating, and quality controlling the data gathered during clinical trials. The goal of CDM is to ensure the data supports conclusions drawn from the research.