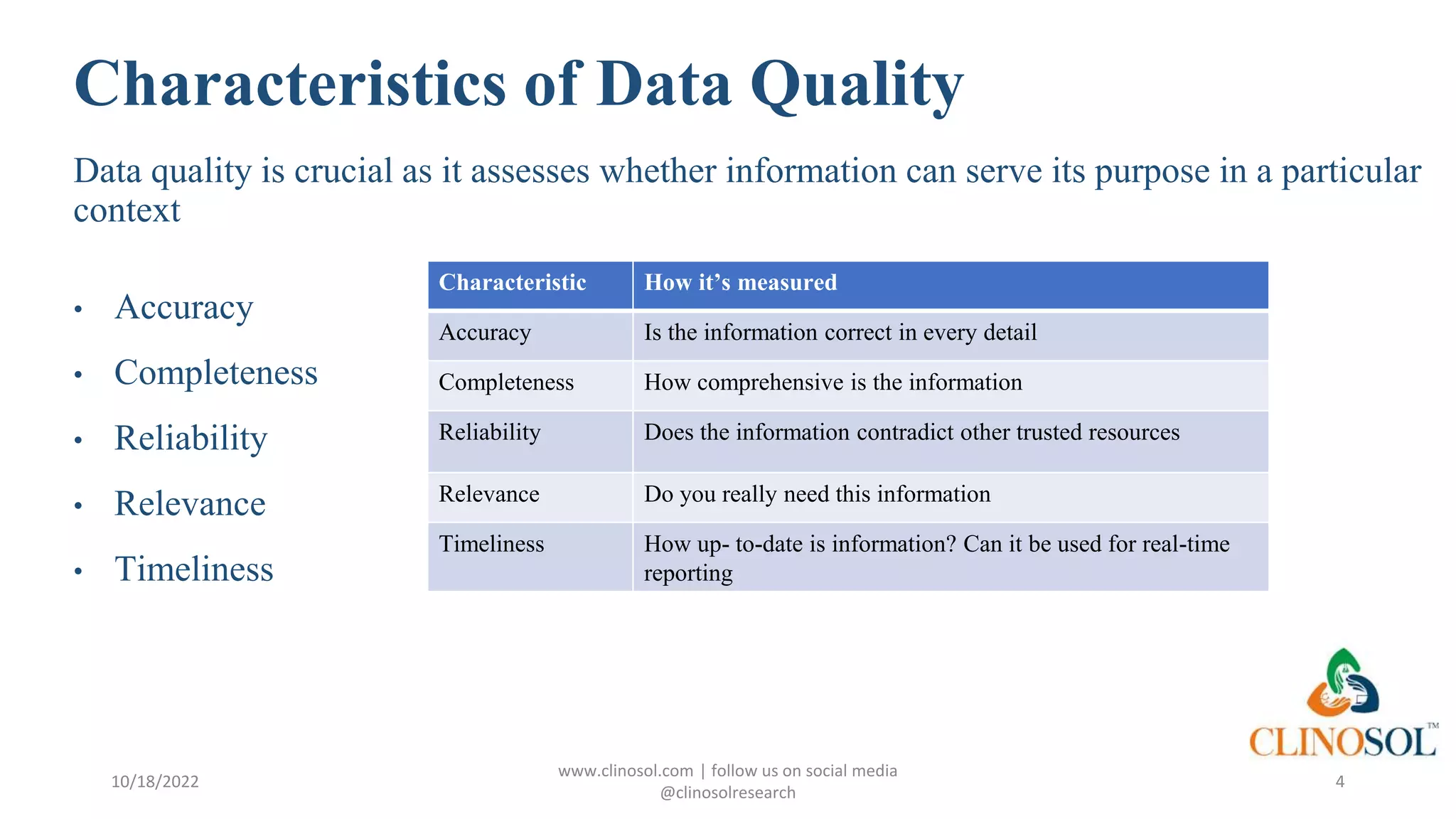
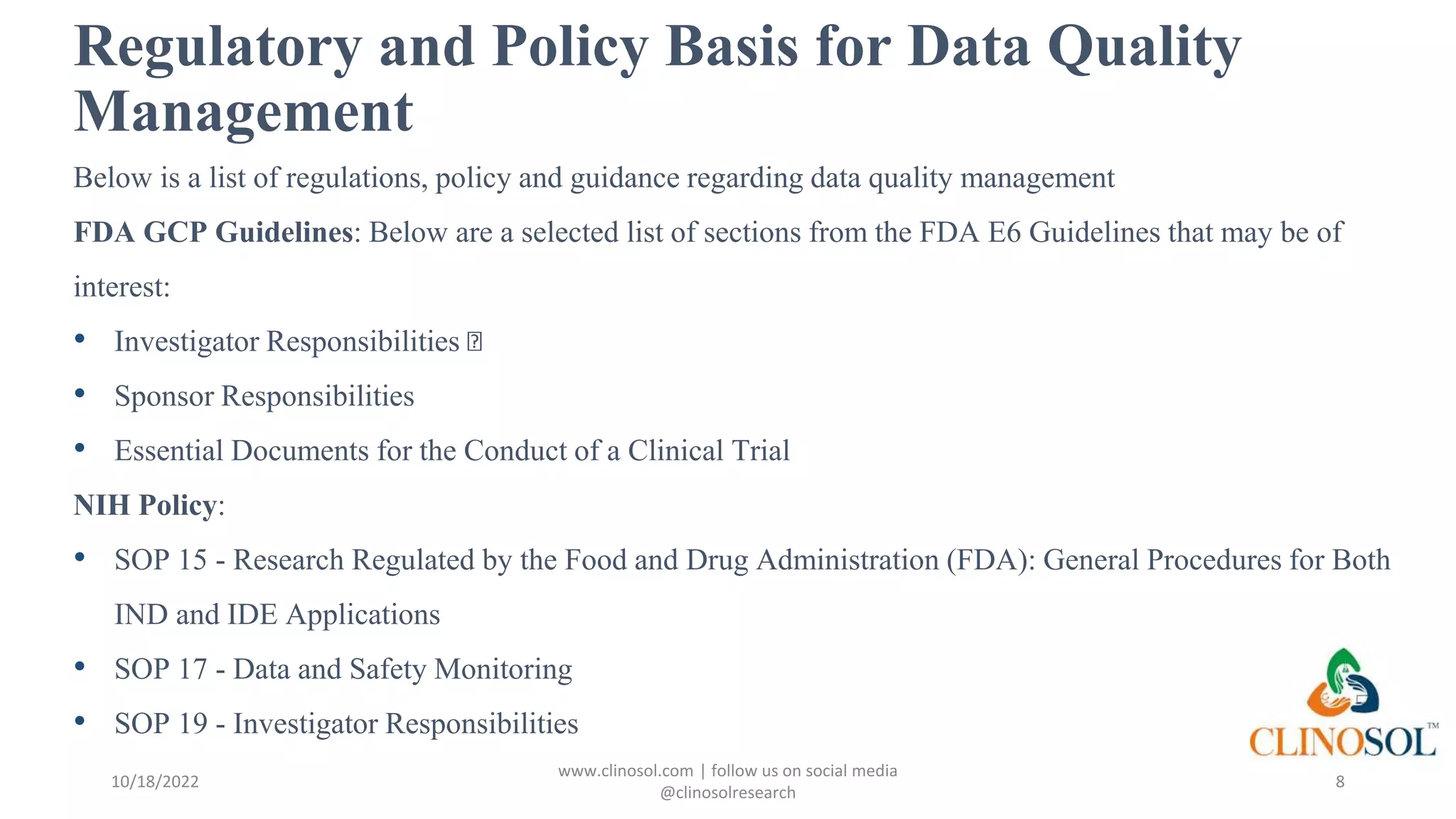
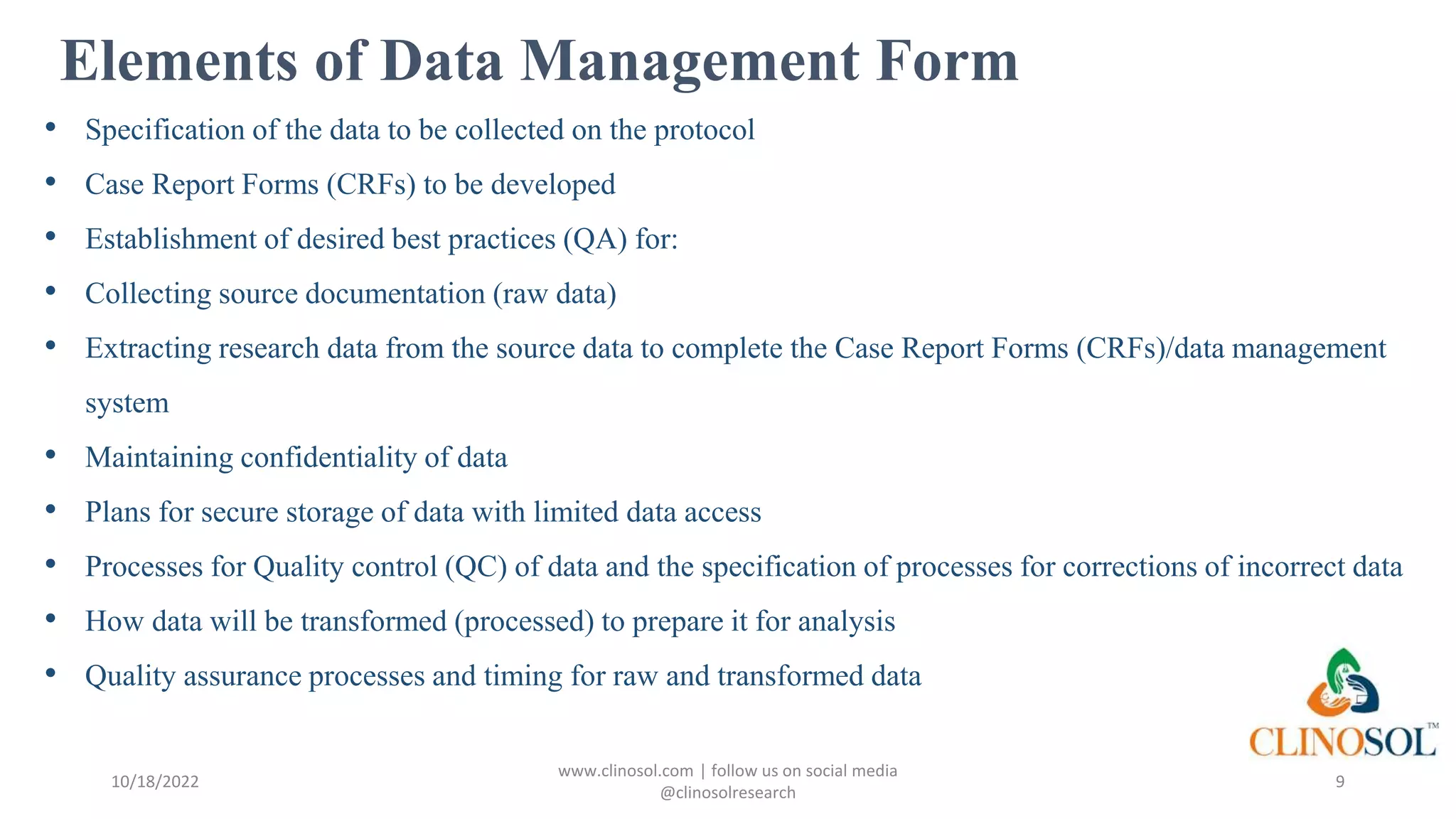
The document outlines best practices and quality control measures for data quality management (DQM) in clinical trials, emphasizing the necessity of accurate, reliable, and timely data to support research conclusions. Key aspects include the importance of data cleansing, profiling, and adherence to regulatory guidelines such as FDA GCP standards. Continuous monitoring and improvement of data management processes are essential to maintain data integrity and ensure compliance throughout the trial.