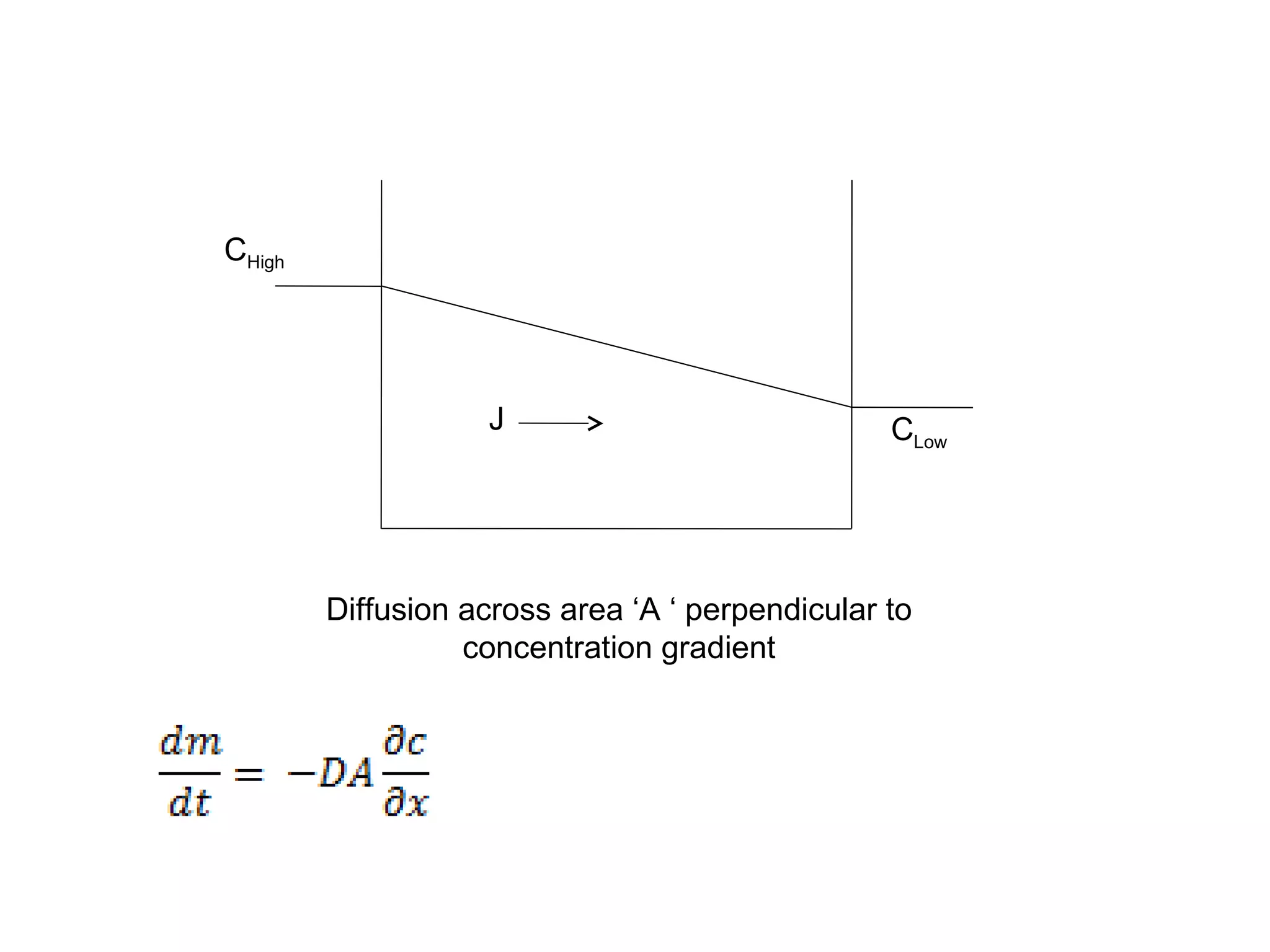
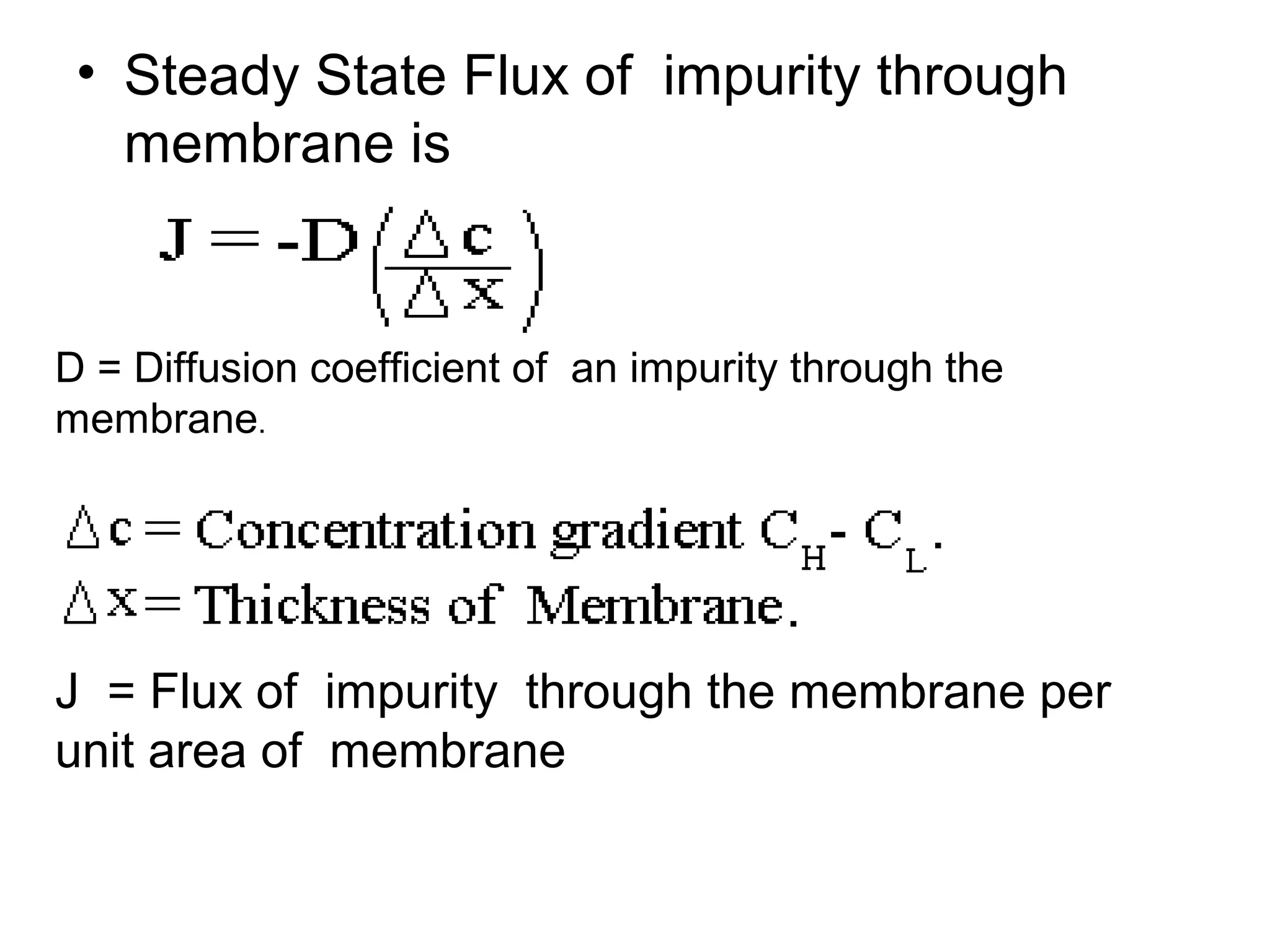
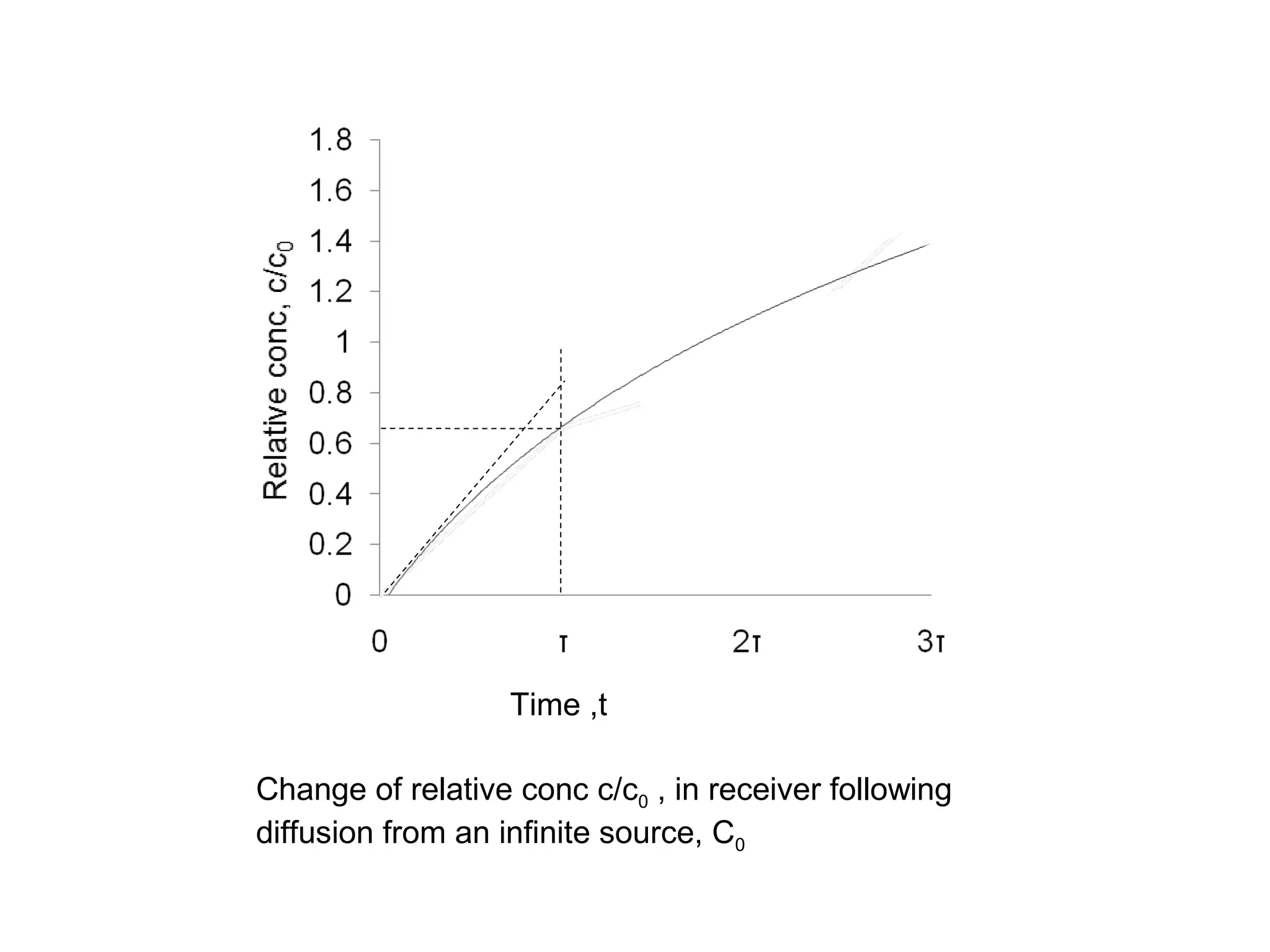
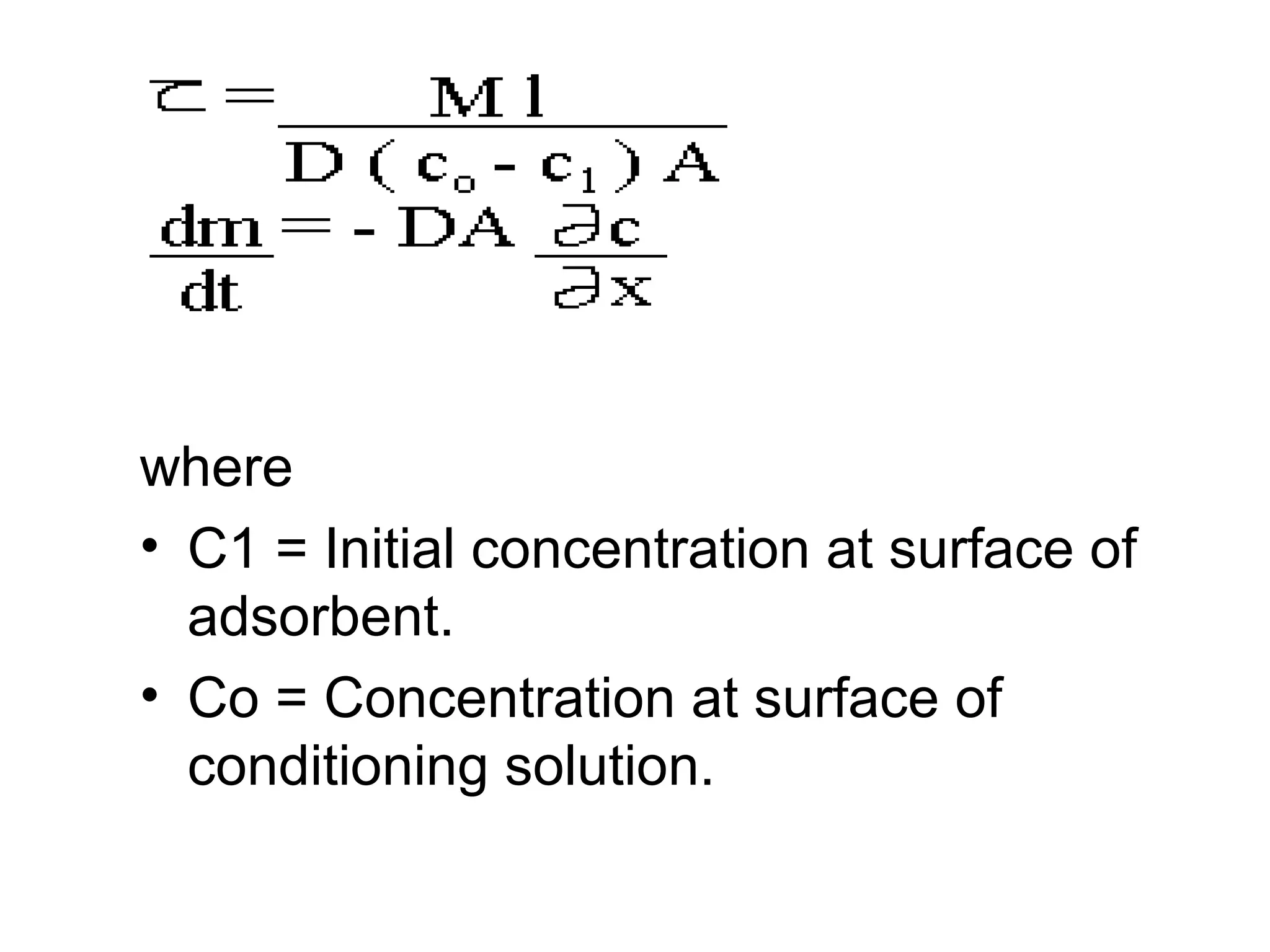
This document discusses the absorption of moisture by different fibers. It explains that the rate of moisture absorption depends on factors like temperature, humidity, thickness, and fiber type. The absorption process involves the slow diffusion of water molecules through the fiber over time until equilibrium is reached. This diffusion is modeled using Fick's laws of diffusion. Fick's first law states that the rate of diffusion is proportional to the concentration gradient, while Fick's second law accounts for how concentration changes over time as moisture penetrates the fiber. The document provides examples of using these laws to analyze moisture absorption in fibers and other materials.