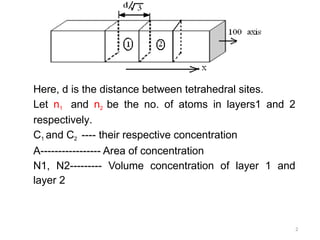
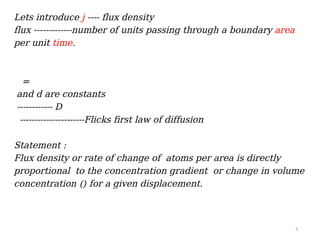
Fick's first law of diffusion describes the relationship between the rate of solute transfer and concentration gradient, stating that the flux density is proportional to the gradient, with diffusivity as a constant. The law has limitations, prompting the development of Fick's second law to account for measurable quantities and changes in flux density over time and distance. Fick's second law elaborates that the rate of change of volume concentration is proportional to the derivative of the concentration gradient.