This document summarizes key aspects of diffusion in solids:
1) Diffusion occurs due to a concentration gradient, which provides a driving force for atoms to move from high to low concentration regions.
2) Diffusion is an important part of materials processing techniques like case hardening and doping of semiconductors.
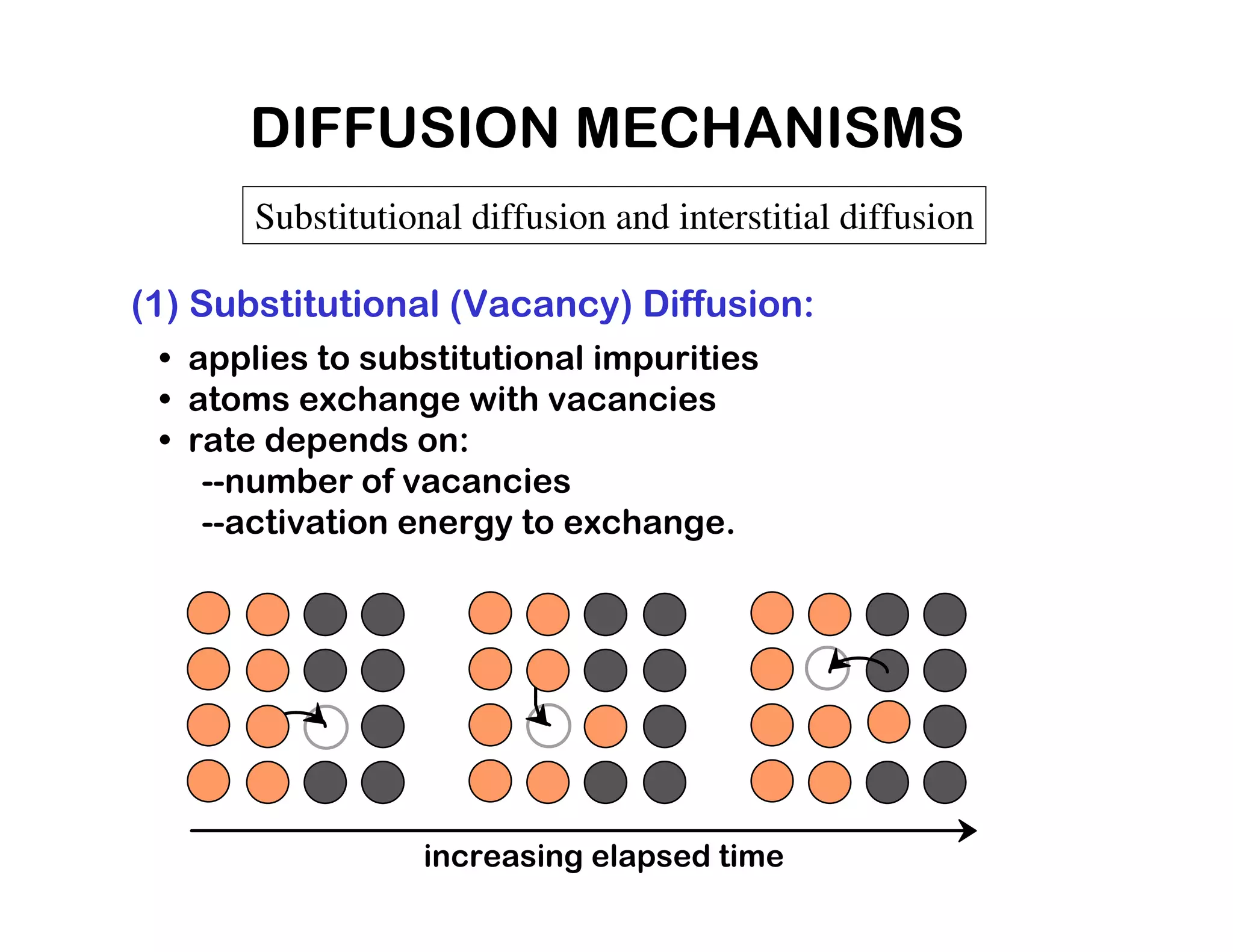
3) The rate of diffusion depends on factors like temperature, structure, and the diffusion mechanism (e.g. substitutional vs. interstitial). Fick's laws can be used to model and predict diffusion rates.











![DIFFUSION AND TEMPERATURE
• Diffusivity increases with T.
pre-exponential [m2/s] (see Table 5.2, Callister 6e)
activation energy
Q [J/mol],[eV/mol]
diffusivity D = Do exp − d (see Table 5.2, Callister 6e )
RT
gas constant [8.31J/mol-K]
• Experimental Data:
1500
1000
600
300
T(C)
10-8 D has exp. dependence on T
C
in
D (m2/s) Ci Recall: Vacancy does also!
γ-
nα
Fe
-Fe Dinterstitial >> Dsubstitutional
10-14 C in α-Fe Cu in Cu
Zn
Al in Al
Fe
C in γ-Fe
in Cun α-
Al
Fe in α-Fe
Cu in Fe
Fe -Fe
in
in
Fe in γ-Fe
Al
γ
i
Zn in Cu
Cu
10-20
0.5 1.0 1.5 2.0 1000K/T](https://image.slidesharecdn.com/chapter06-1280669199-phpapp01/75/Chapter-06-14-2048.jpg)





