This document discusses electromagnetic radiation and its wave-particle duality. It covers key topics such as:
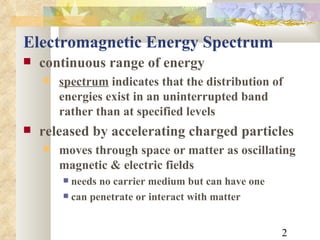
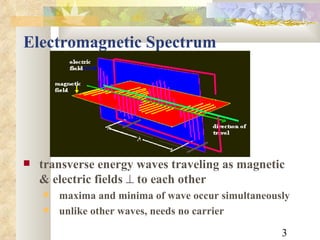
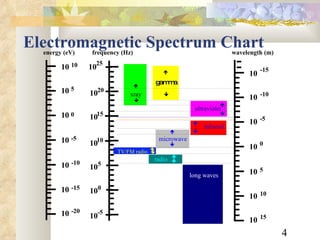
1) The electromagnetic spectrum ranges from radio waves to gamma rays, with different wavelengths and frequencies. Shorter wavelengths correspond to higher frequencies and energies.
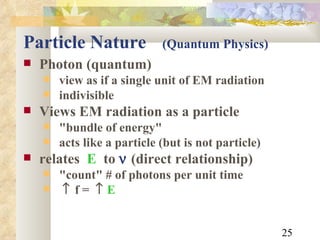
2) Electromagnetic waves exhibit both wave and particle properties. The particle view is that radiation consists of discrete photon packets with energy directly proportional to frequency.
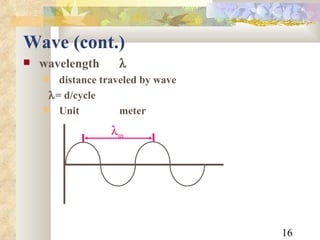
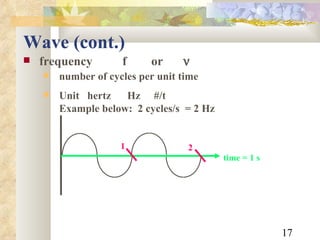
3) Key relationships allow converting between wavelength, frequency, and photon energy, using constants like Planck's constant and the speed of light. Shortcut formulae allow quick calculation of photon wavelength from energy in keV or vice versa.









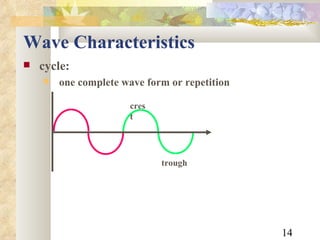
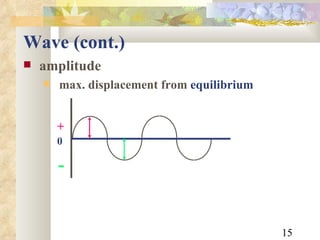


![Example An x-ray photon has a wavelength of 2.1nm. What is its frequency? f = 2.1x10 -9 m c = 3x10 8 m/s c = f f = c / = [3x10 8 m/s] / [2.1x10 -9 m] = 1.428571428571 x 10 17 /s = 1.4 x 10 17 Hz](https://image.slidesharecdn.com/radiationphysics-100510231438-phpapp01/85/Radiation-physics-22-320.jpg)
![Example #2 A radio station broadcasts at 104.5 MHz. What Is the wavelength of the broadcast? = ?? 104.5 x 10 6 /s = [c = 3 x 10 8 m/s] c = f = c / f = [3 x 10 8 m/s] / [104.5 x 10 6 /s] = 0.028708134 x 10 2 m = 2.871 m](https://image.slidesharecdn.com/radiationphysics-100510231438-phpapp01/85/Radiation-physics-23-320.jpg)
![Example #3 What it the frequency of microwave radiation that has a wavelength of 10 -4 m? f = ?? 1 x 10 -4 m = [c = 3 x 10 8 m/s] c = f = c / = [3 x 10 8 m/s] / [1 x 10 -4 m ] = 3 x 10 12 Hz](https://image.slidesharecdn.com/radiationphysics-100510231438-phpapp01/85/Radiation-physics-24-320.jpg)
![Mathematics E f E = h f h = Planck’s constant = 4.15 x 10 -15 eVs units usual energy units = J EM energy units = variation of J [eVs][/s] = eV x rays & gamma rays usually in keV or MeV](https://image.slidesharecdn.com/radiationphysics-100510231438-phpapp01/85/Radiation-physics-26-320.jpg)
![Example What is the energy (keV) of an x-ray photon with a frequency of 1.6 x 10 19 Hz? E = ?? 1.6x10 19 Hz = f [h = 4.15 x 10 -15 eVs] E = h f = [ 4.15 x 10 -15 eVs] [ 1.6 x 10 19 Hz] = 6.64 x 10 4 eV = [6.64 x 10 4 eV] / [10 3 ev / keV ] = 6.64 x10 1 keV = 66 keV](https://image.slidesharecdn.com/radiationphysics-100510231438-phpapp01/85/Radiation-physics-27-320.jpg)
![Example #2 What is the energy in MeV of an x-ray photon with a frequency of 2.85 x 10 21 Hz? E = ?? 2.85x10 21 Hz = f [h = 4.15 x 10 -15 eVs] E = h f = [4.15 x 10 -15 eVs] [2.85 x 10 21 Hz] = 11.8275 x 10 6 eV = [11.8275 x 10 6 eV] / [10 6 ev / MeV ] = 11.8 MeV](https://image.slidesharecdn.com/radiationphysics-100510231438-phpapp01/85/Radiation-physics-28-320.jpg)

![Combination of Wave & Practical Theories combine formulas: c = E = h solve wave wave equation for frequency: = c / insert solution in quantum formula: [4.15 x 10 -15 eVs] [3 x 10 8 m/s] m [12.4 x 10 -7 eVm] m hc m E eV =](https://image.slidesharecdn.com/radiationphysics-100510231438-phpapp01/85/Radiation-physics-30-320.jpg)
![Shortcut Formulae E eV = hc/ = [12.4x10 -7 eVm] / m by incorporating changes in prefixes you can arrive at the following shortcut formulae: nm = 10 -9 m [12.4 keV A] A E keV = A = 10 -10 m [1.24 keVnm] nm E keV =](https://image.slidesharecdn.com/radiationphysics-100510231438-phpapp01/85/Radiation-physics-31-320.jpg)
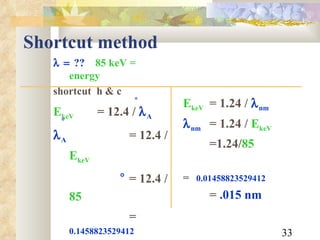
![Example What is the of an 85 keV x-ray photon? ?? 85 keV = energy need h & c E eV = hc m m = [4.15 x 10 -15 eVs] [3 x 10 8 m/s] E eV = [12.4x10 -7 eVm] 85 x 10 3 eV = 0.1458823529412 x 10 -10 m = .15 x 10 -10 m or .15A ](https://image.slidesharecdn.com/radiationphysics-100510231438-phpapp01/85/Radiation-physics-32-320.jpg)


![Relativity problem example: What is the matter equivalence of a 86keV x-ray photon? ? = mass E = 86keV [c = 3x10 8 m/s]](https://image.slidesharecdn.com/radiationphysics-100510231438-phpapp01/85/Radiation-physics-36-320.jpg)
![Relativity problem example: How many electron-volts are contained in .25kg of matter? ? = E m = .25 kg [c = 3x10 8 m/s]](https://image.slidesharecdn.com/radiationphysics-100510231438-phpapp01/85/Radiation-physics-37-320.jpg)