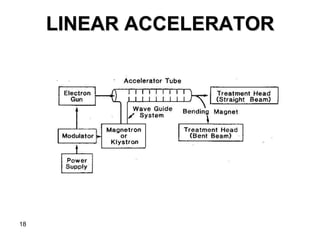
This document discusses various types of clinical radiation generators used for radiation therapy. It describes kilovoltage units that generate x-rays up to 300 kV and various superficial therapies. It also discusses megavoltage therapy using linear accelerators, betatrons, and cobalt-60 units to treat deeper tumors. Various particle beams including neutrons, protons, and pions are also mentioned but noted to still be experimental with high costs.