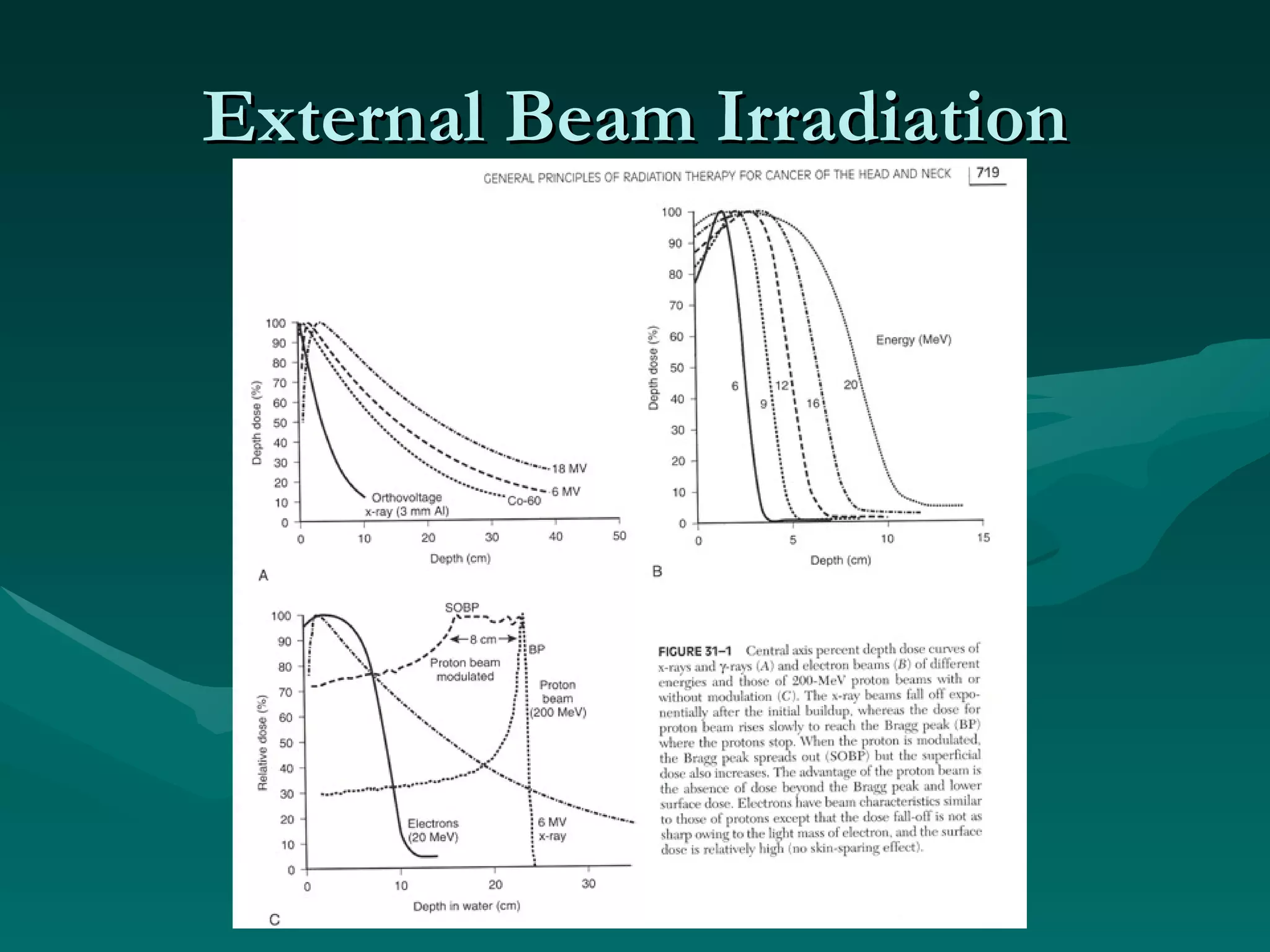
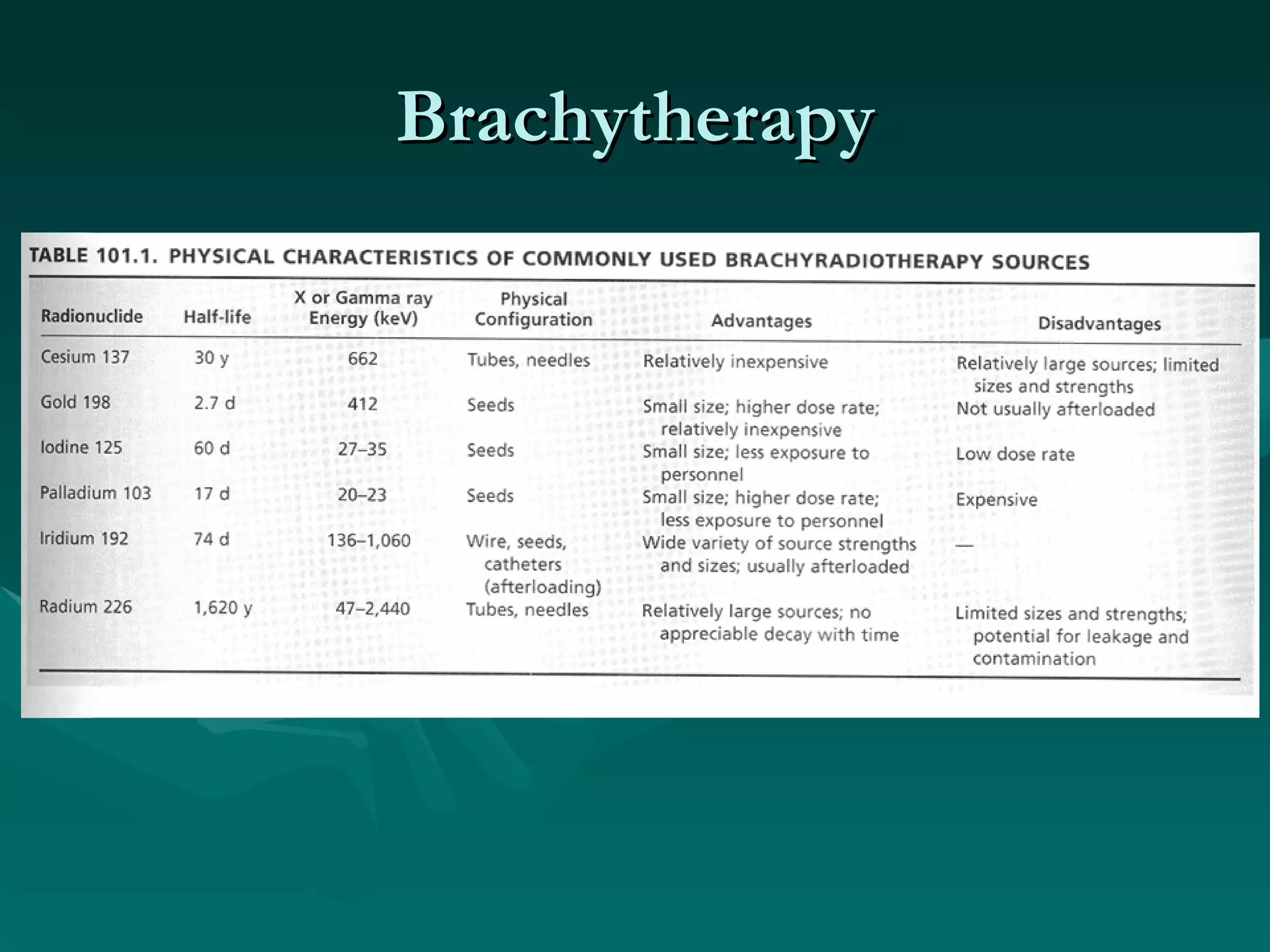
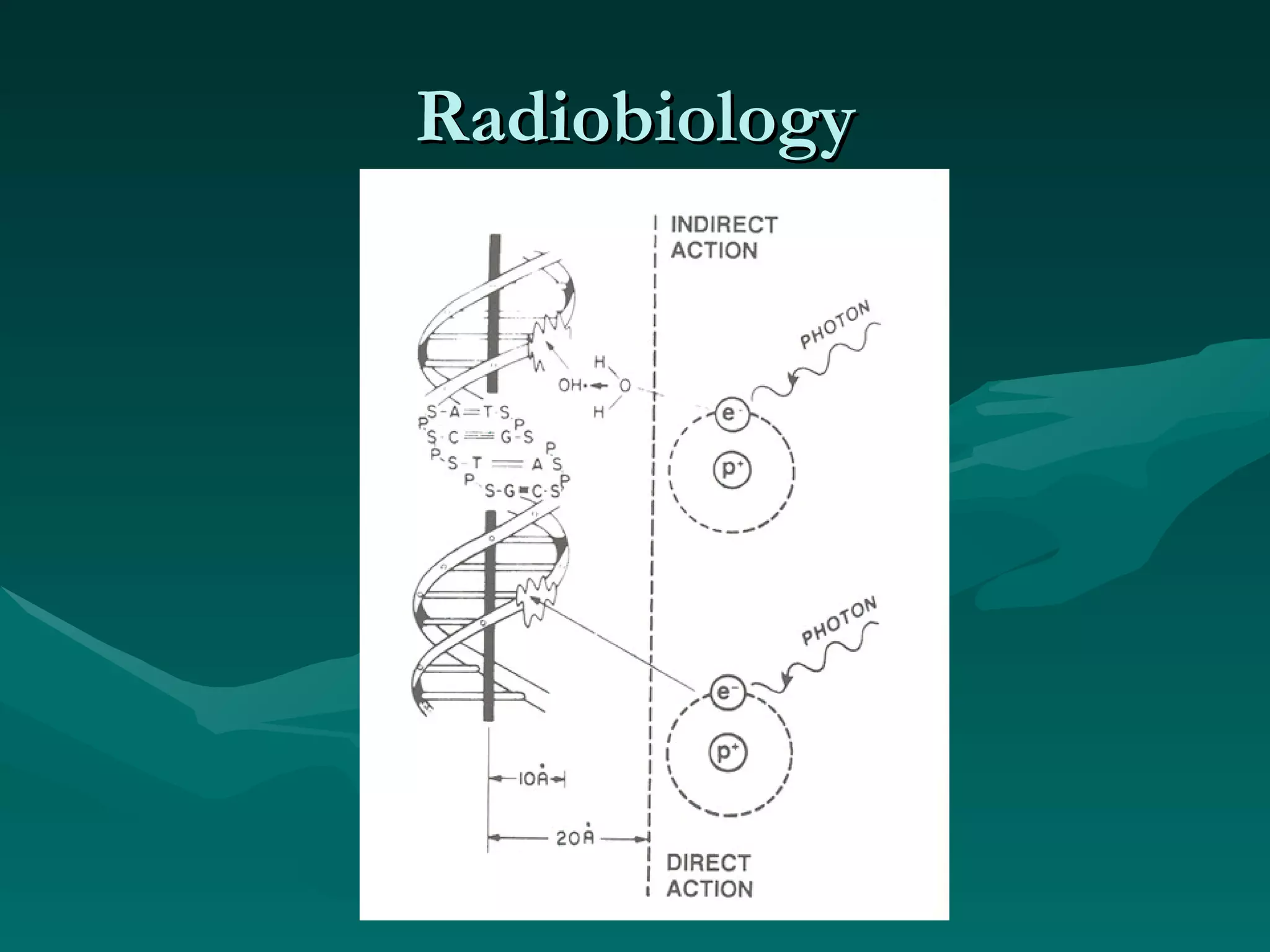
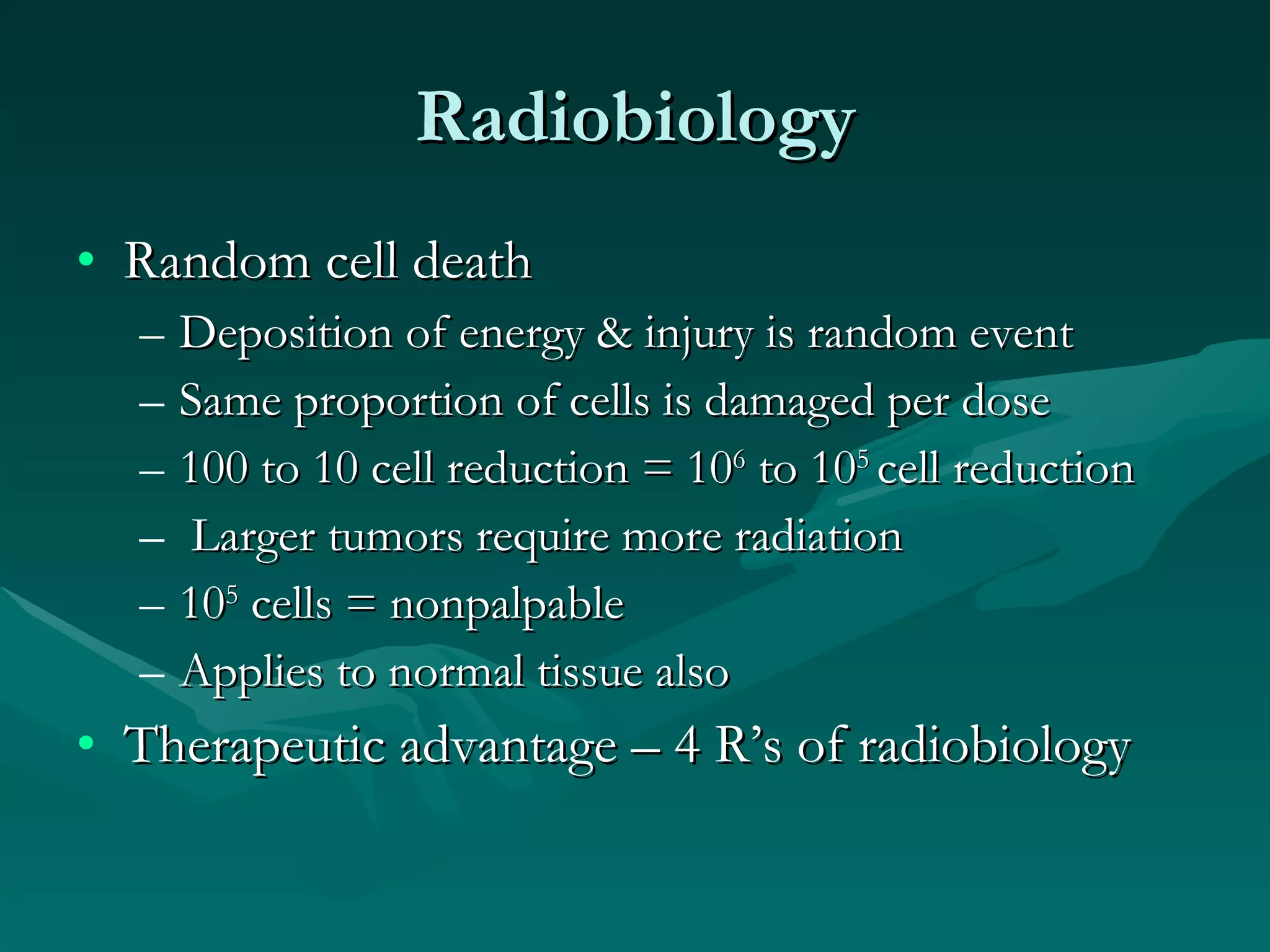
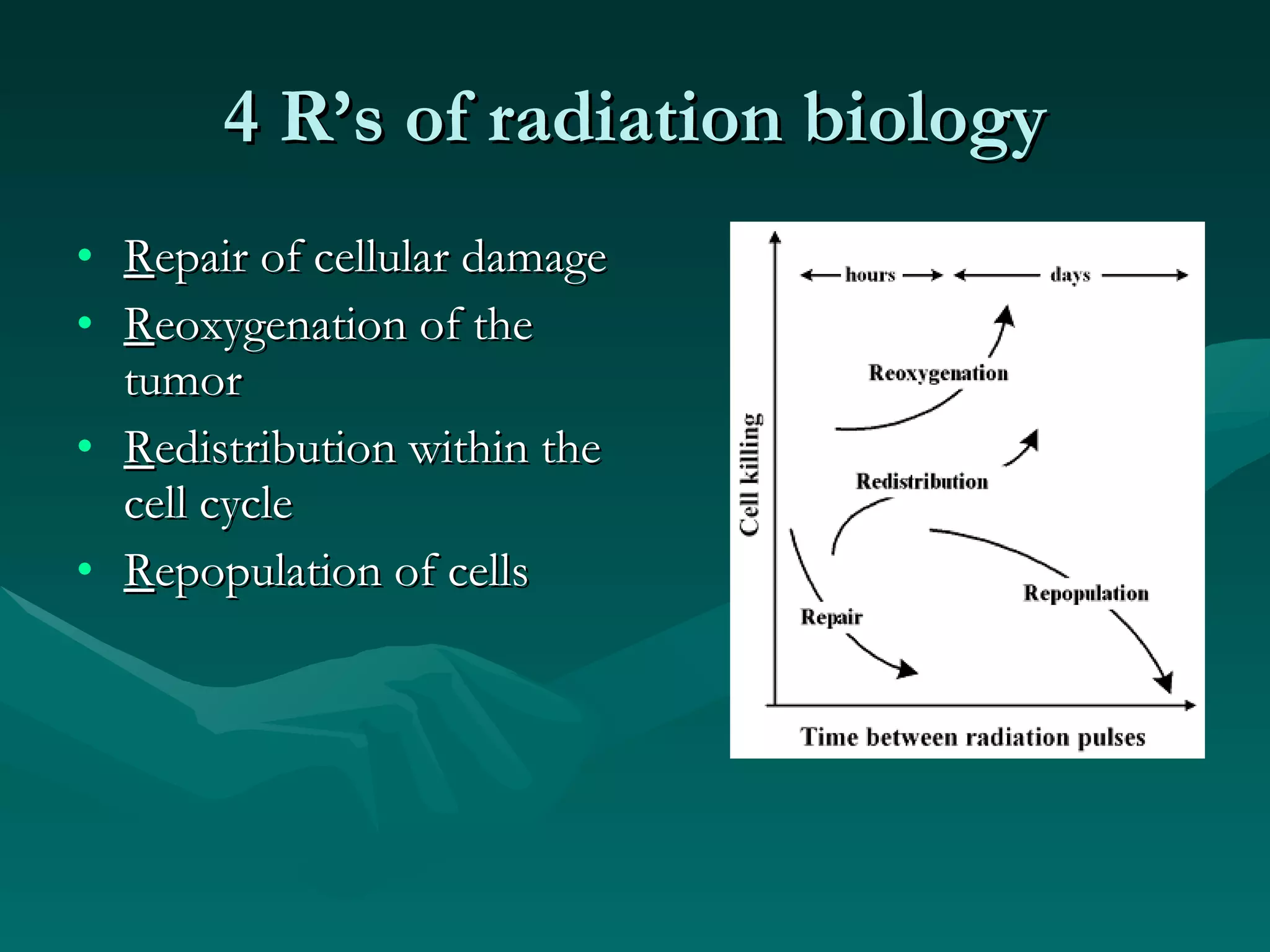
1) The document outlines the basic principles of radiation oncology, including the physics of radiation, external beam irradiation, brachytherapy, and radiobiology concepts like the four R's.
2) It discusses treatment planning considerations for factors like tumor size and location as well as combined modality approaches using surgery and radiation.
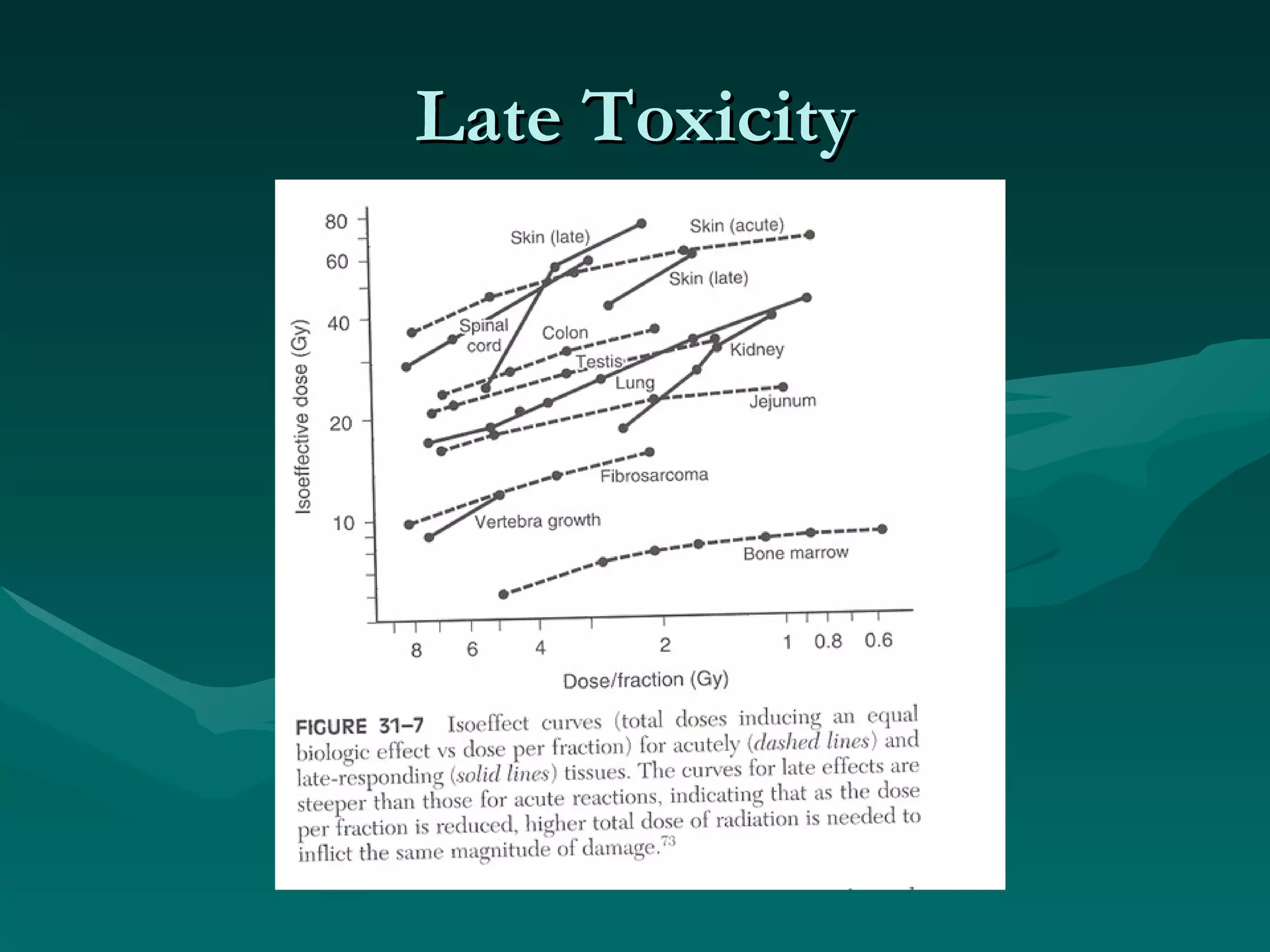
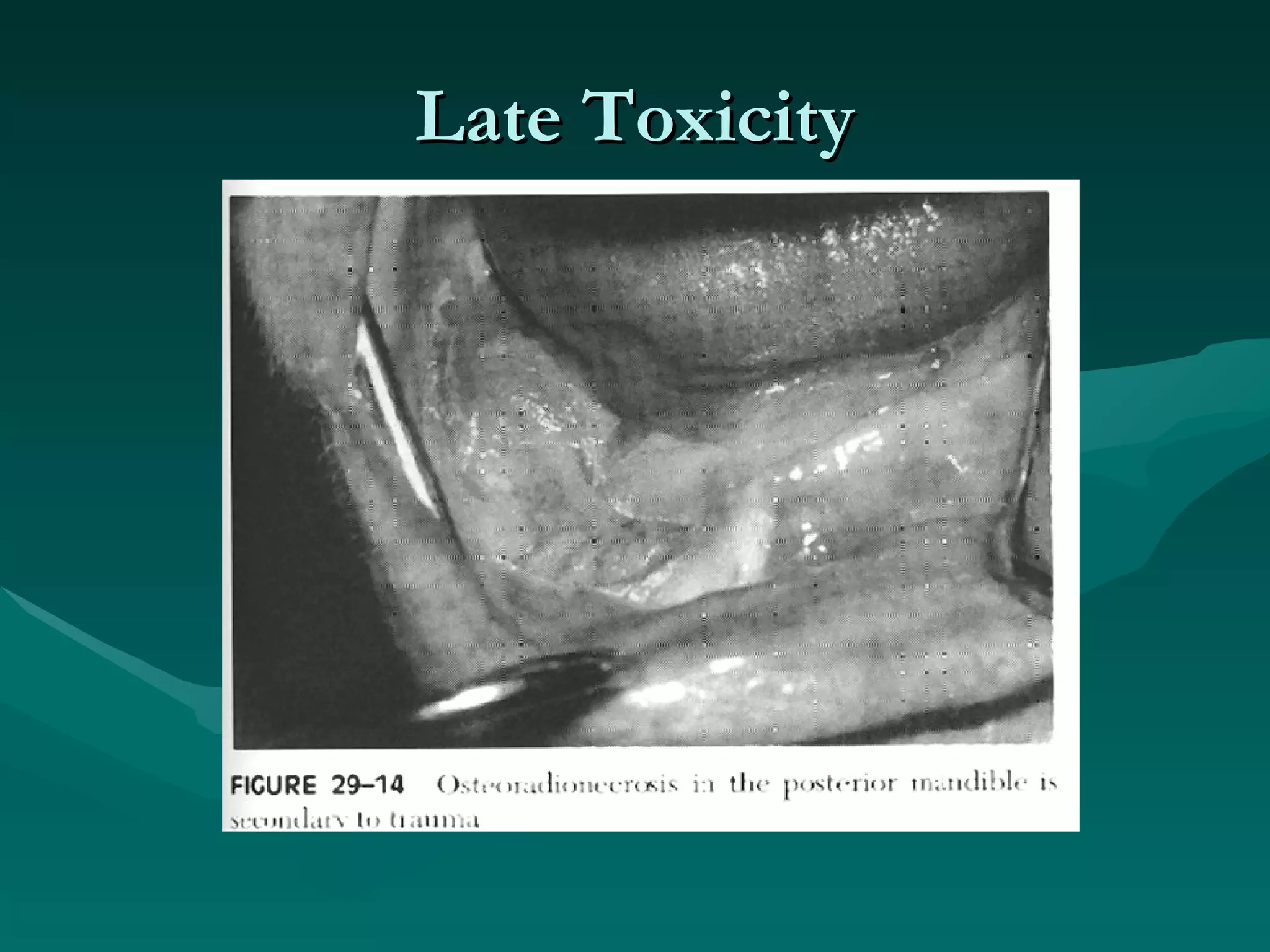
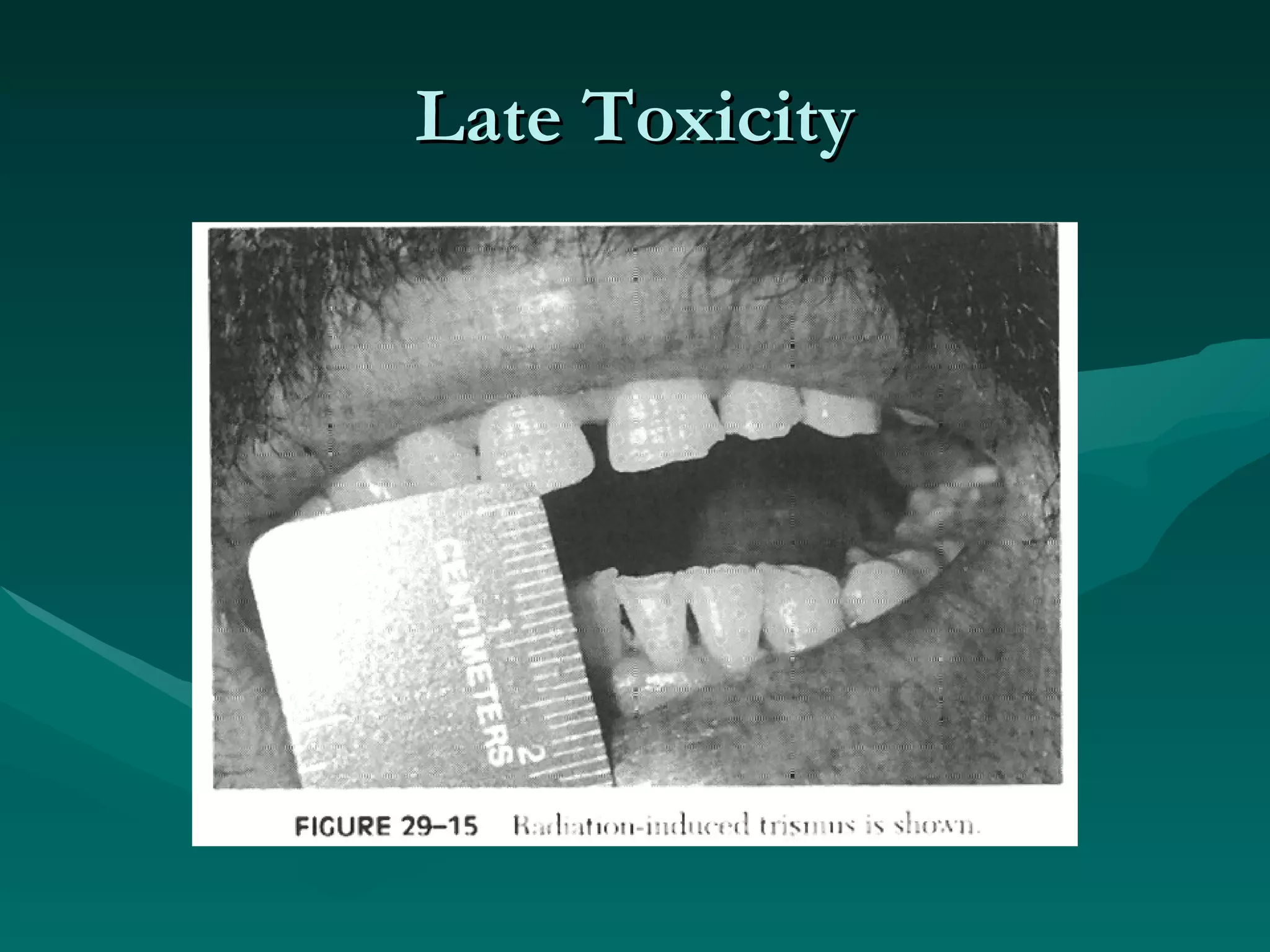
3) Common acute and late complications of radiation therapy are reviewed, such as mucositis, xerostomia, fibrosis, and central nervous system toxicity.