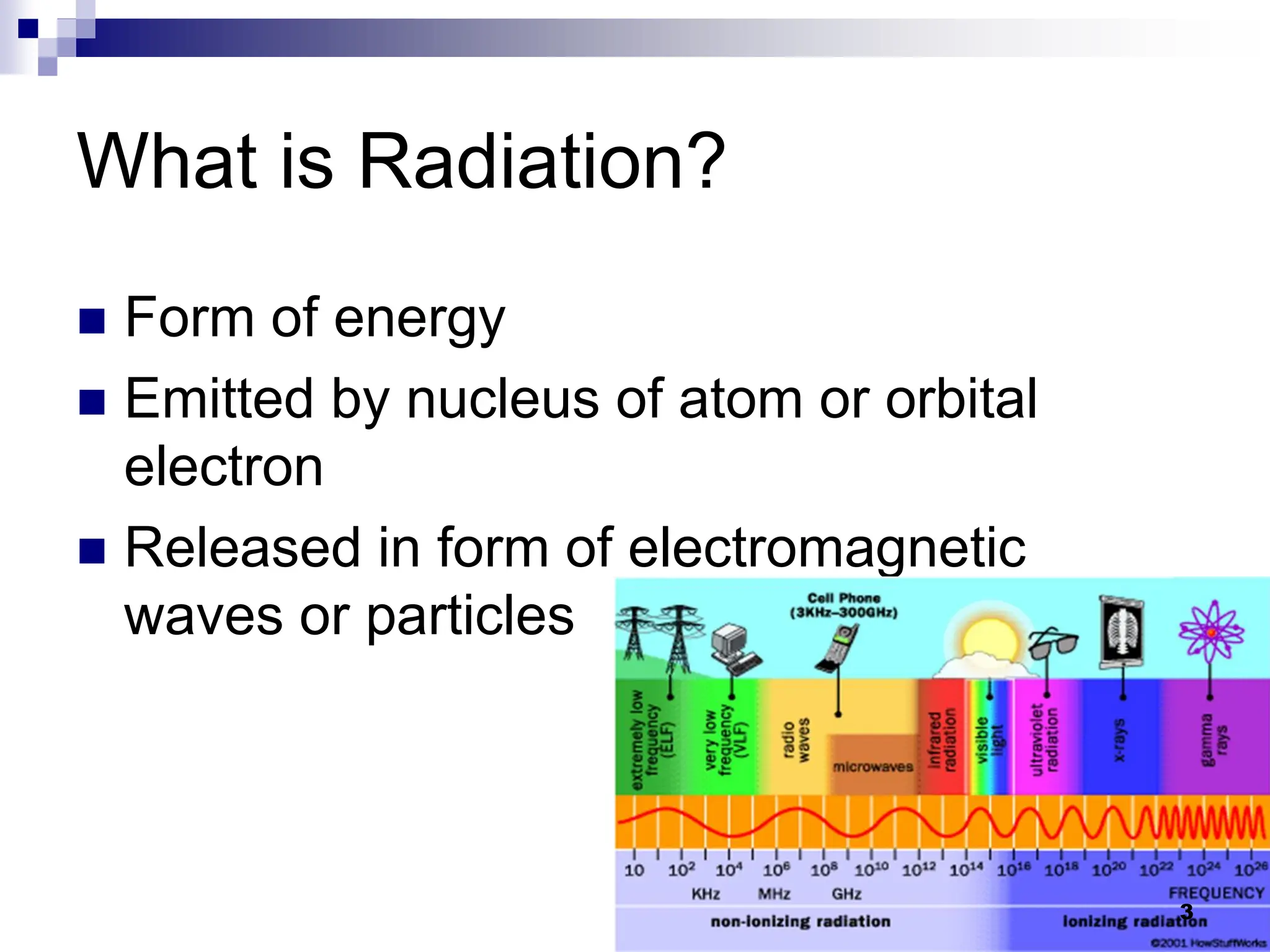
This document provides an overview of biophysics in radiology and nuclear medicine. It discusses the basic physics of ionizing radiation, including electromagnetic waves, atomic structure, nuclear stability, and radioactive decay. It then defines different types of radiation and electromagnetic waves. It explains how moving electric charges create magnetic fields and how electric and magnetic fields change and propagate. It also covers the properties of electromagnetic waves including their universal speed and interactions with matter. Finally, it describes different parts of the electromagnetic spectrum from radio waves to gamma rays and some of their medical applications.







![Characteristics of Waves
Amplitude – x [m]
Wavelength – l [m]
Period -T [s]
Wave Speed – c [m/s]
Frequency – n [1/s=Hz]
9](https://image.slidesharecdn.com/basicphysicsofionizingradiationi-240213071521-5a3f17fa/75/Basic-physics-of-ionizing-radiation-I-pdf-9-2048.jpg)

























