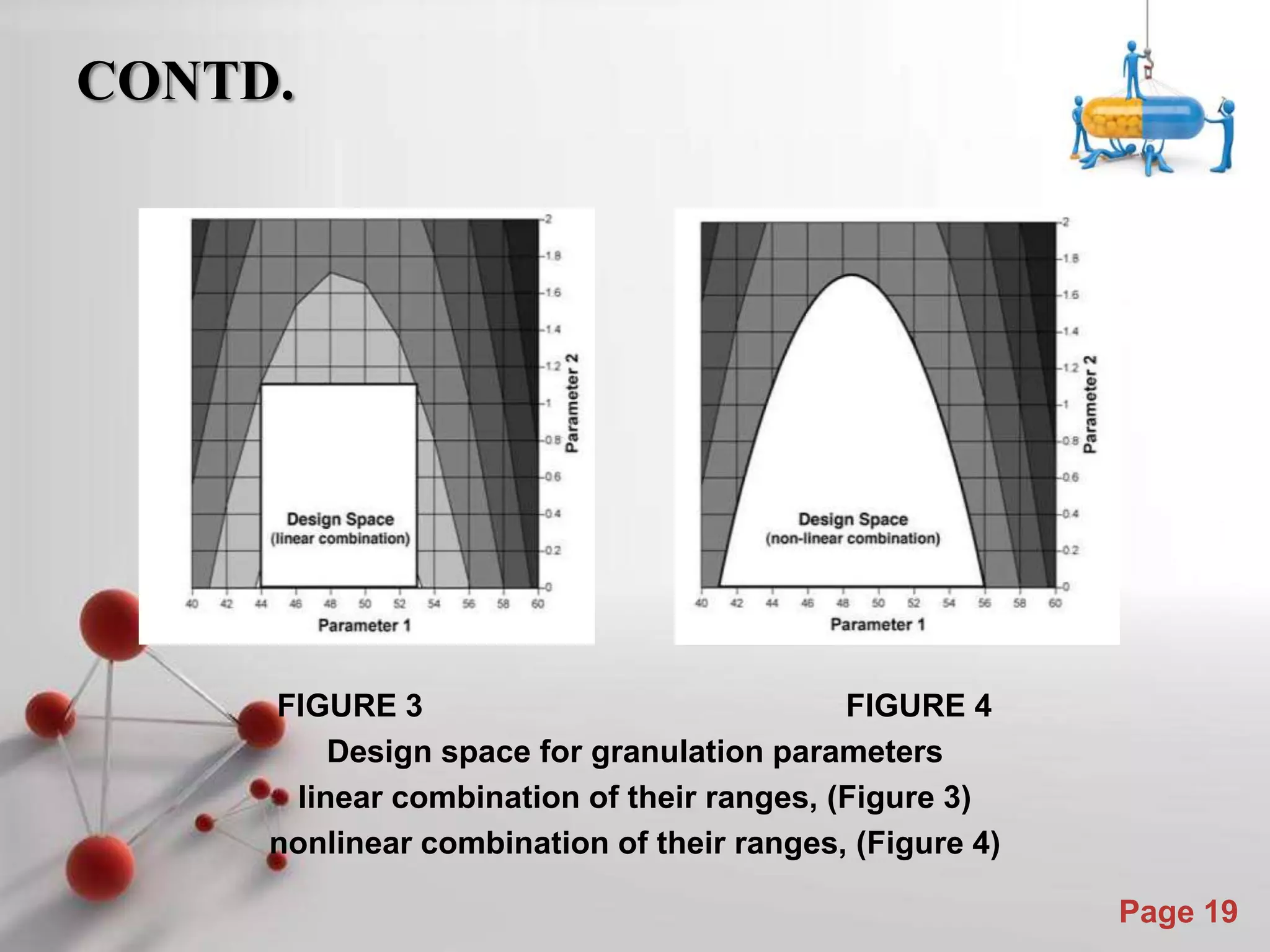
This document discusses the role of Quality by Design (QbD) in assuring quality of pharmaceutical products. It defines QbD and compares the traditional quality assessment system to the QbD approach. The document outlines the steps of a QbD program, including defining target quality profiles, identifying critical quality attributes and process parameters, designing the manufacturing process and establishing a control strategy. It also discusses tools used in QbD like design of experiments and risk assessment.