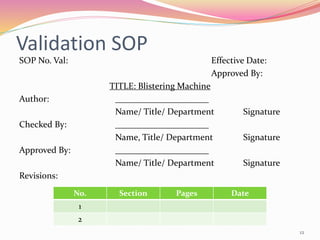
The document discusses validation of packaging machines. It outlines the objectives, importance, and responsibilities of process validation. Validation establishes that a machine meets installation, operational, and performance qualification requirements. The document describes user requirement specifications that cover mandatory parts to guarantee final product quality and compliance. It details the scope, steps in the machine's lifecycle, types of packaging machines, their detailed assembly, key parameters, and the validation SOP.