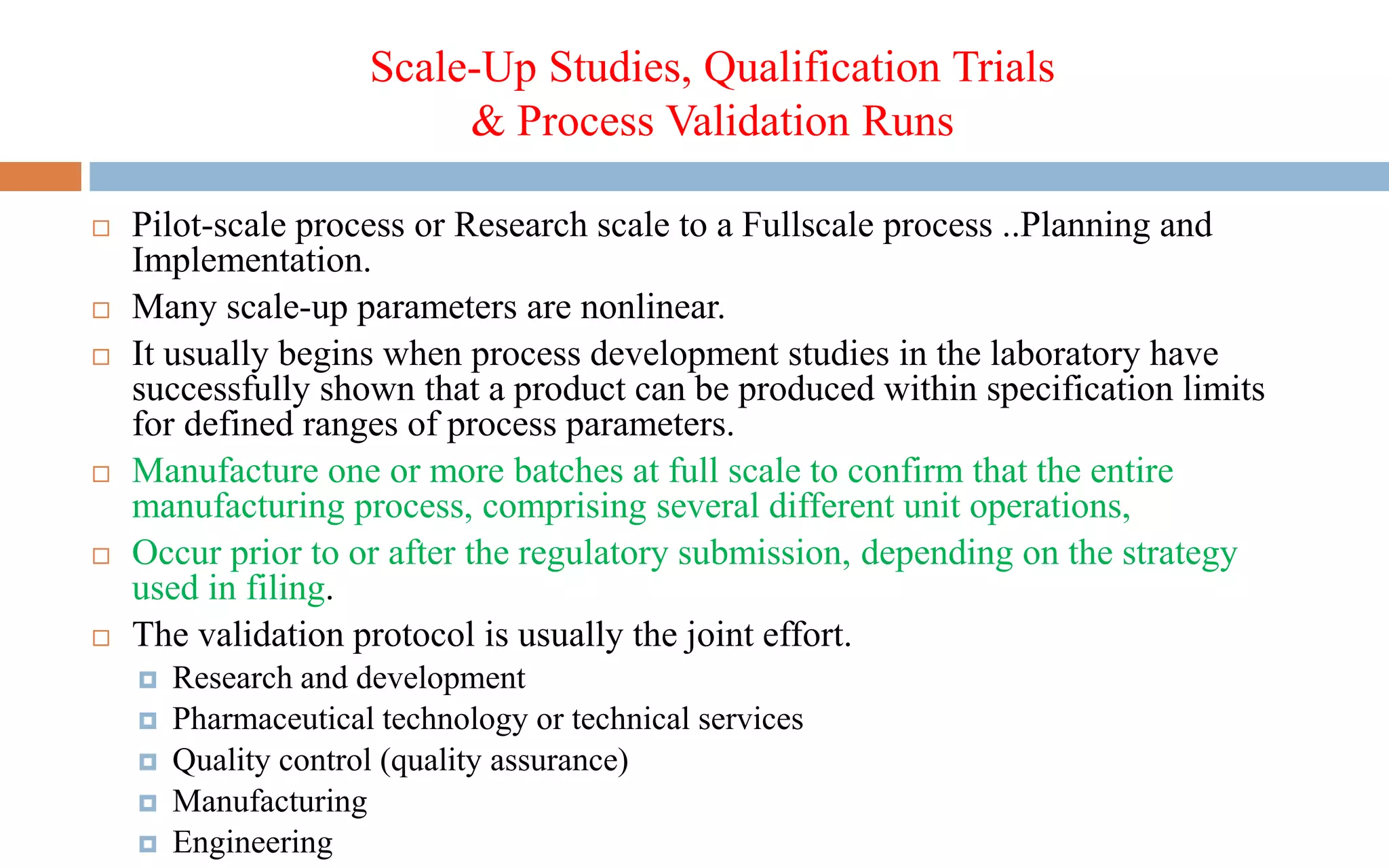
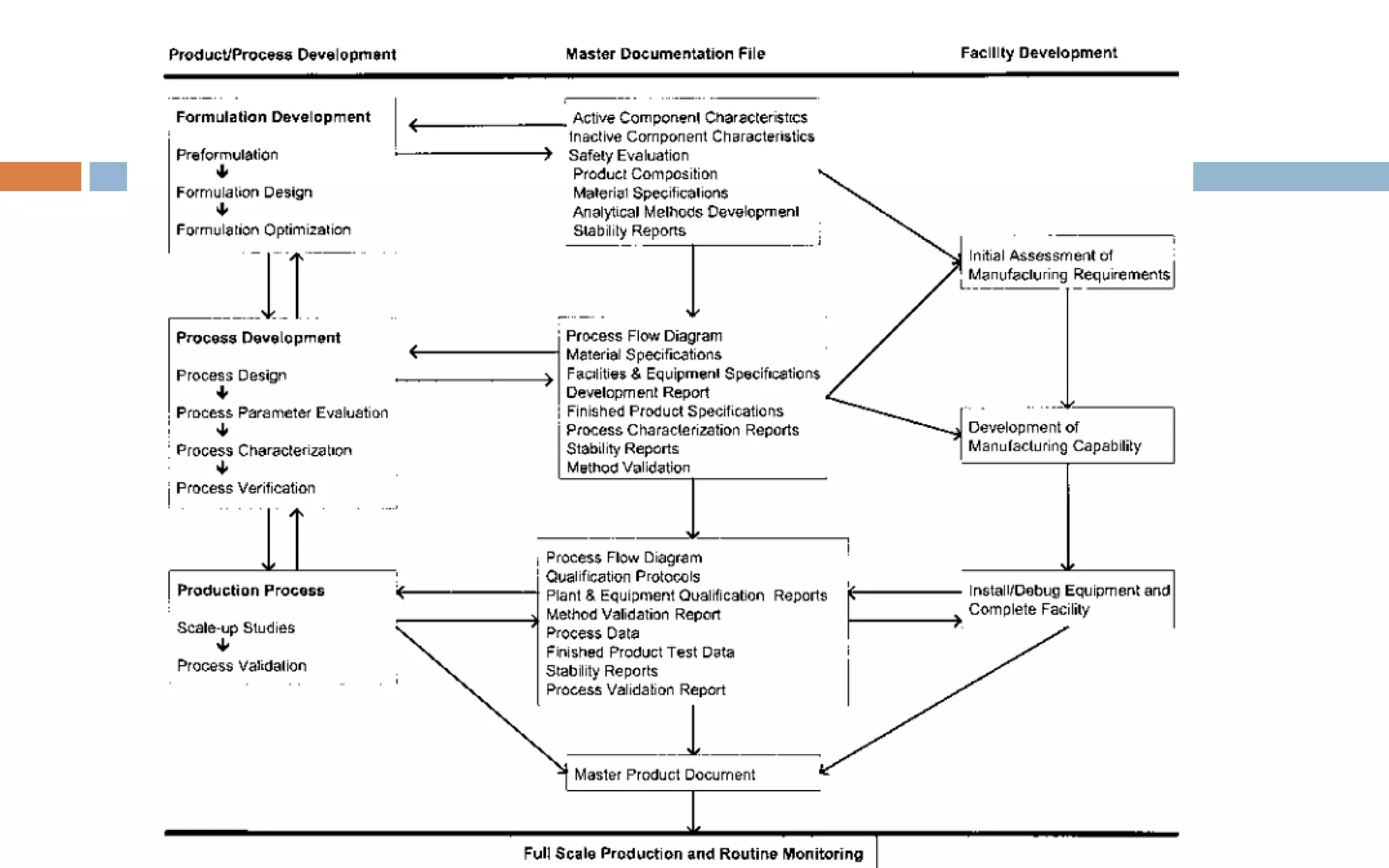
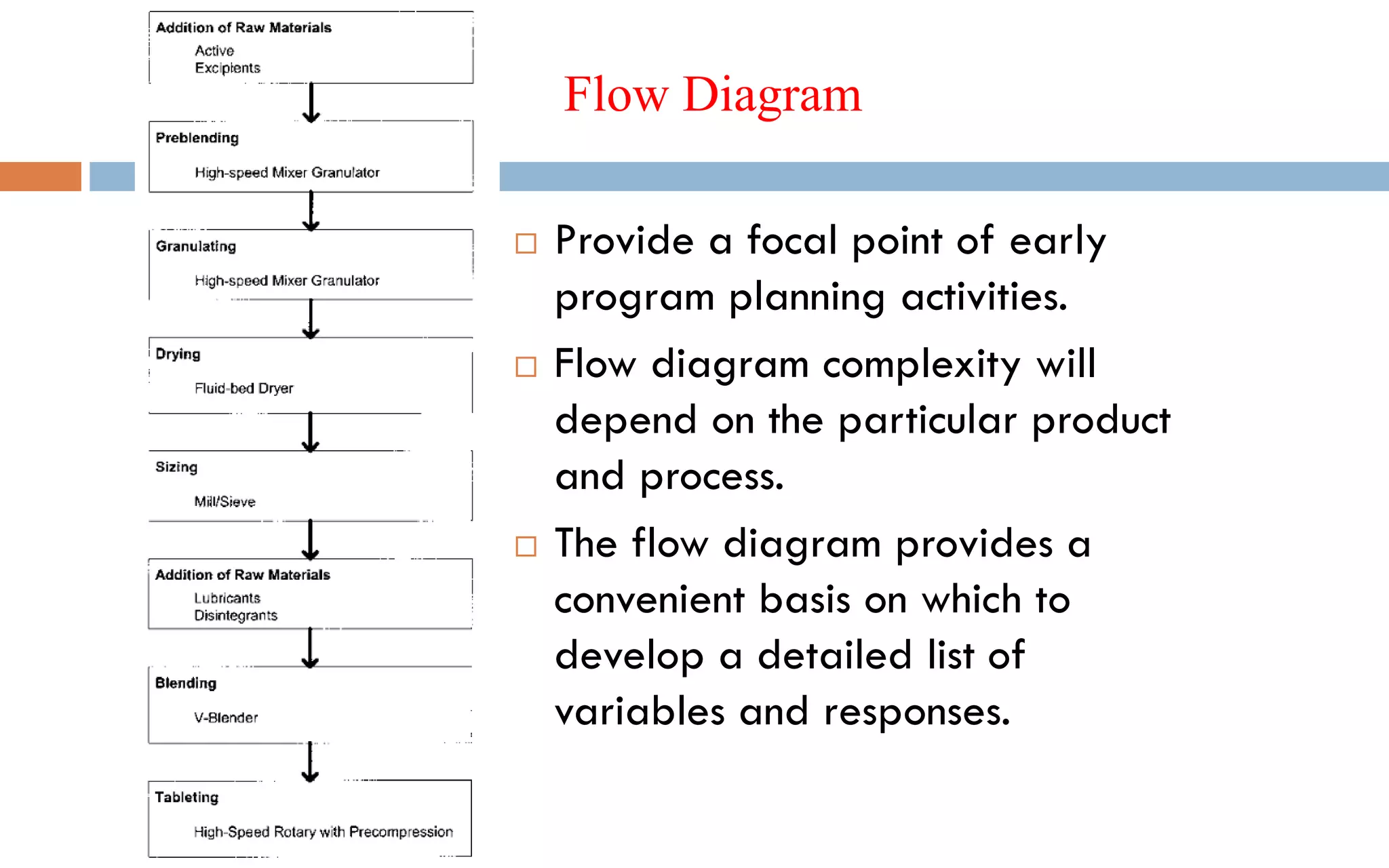
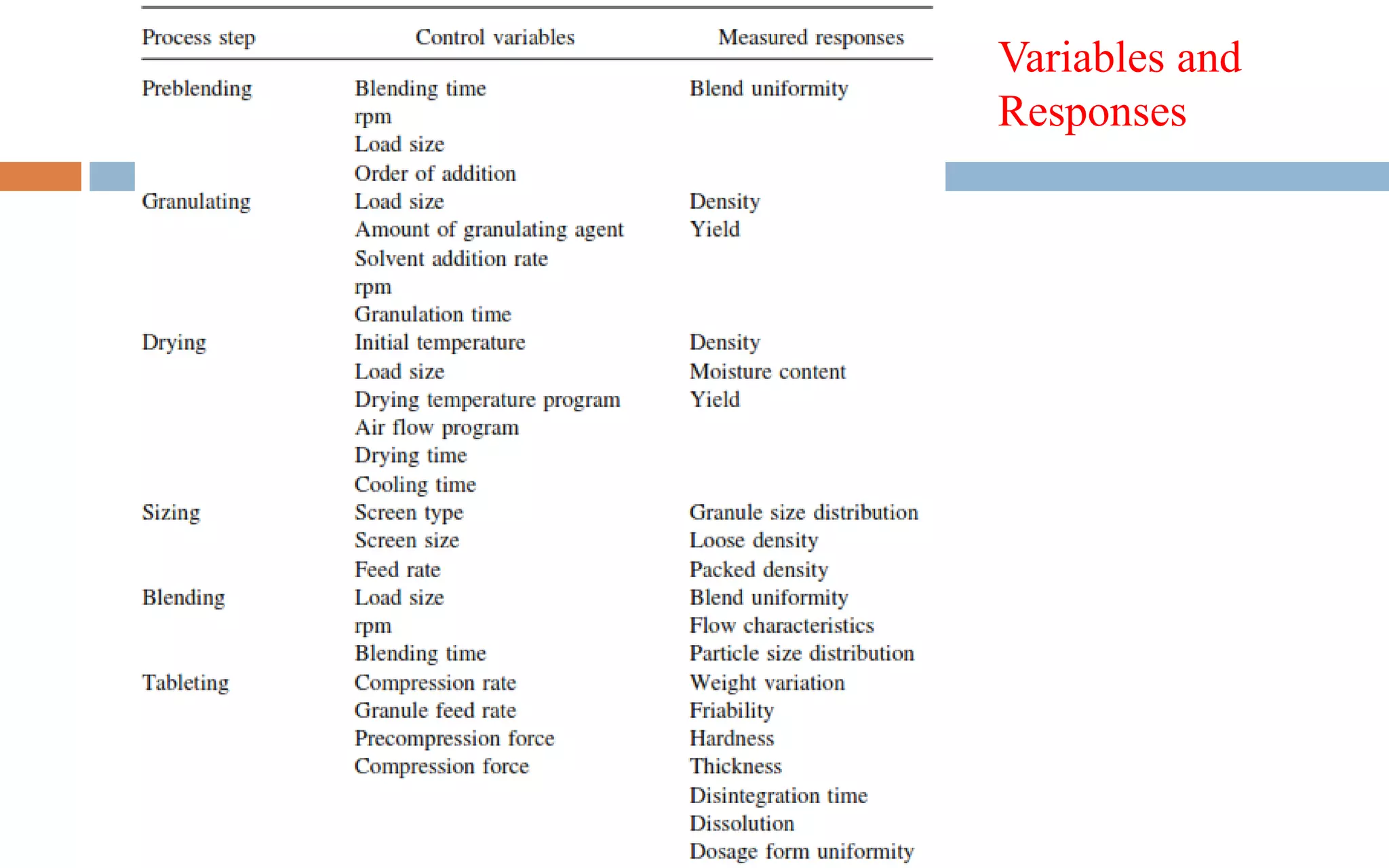
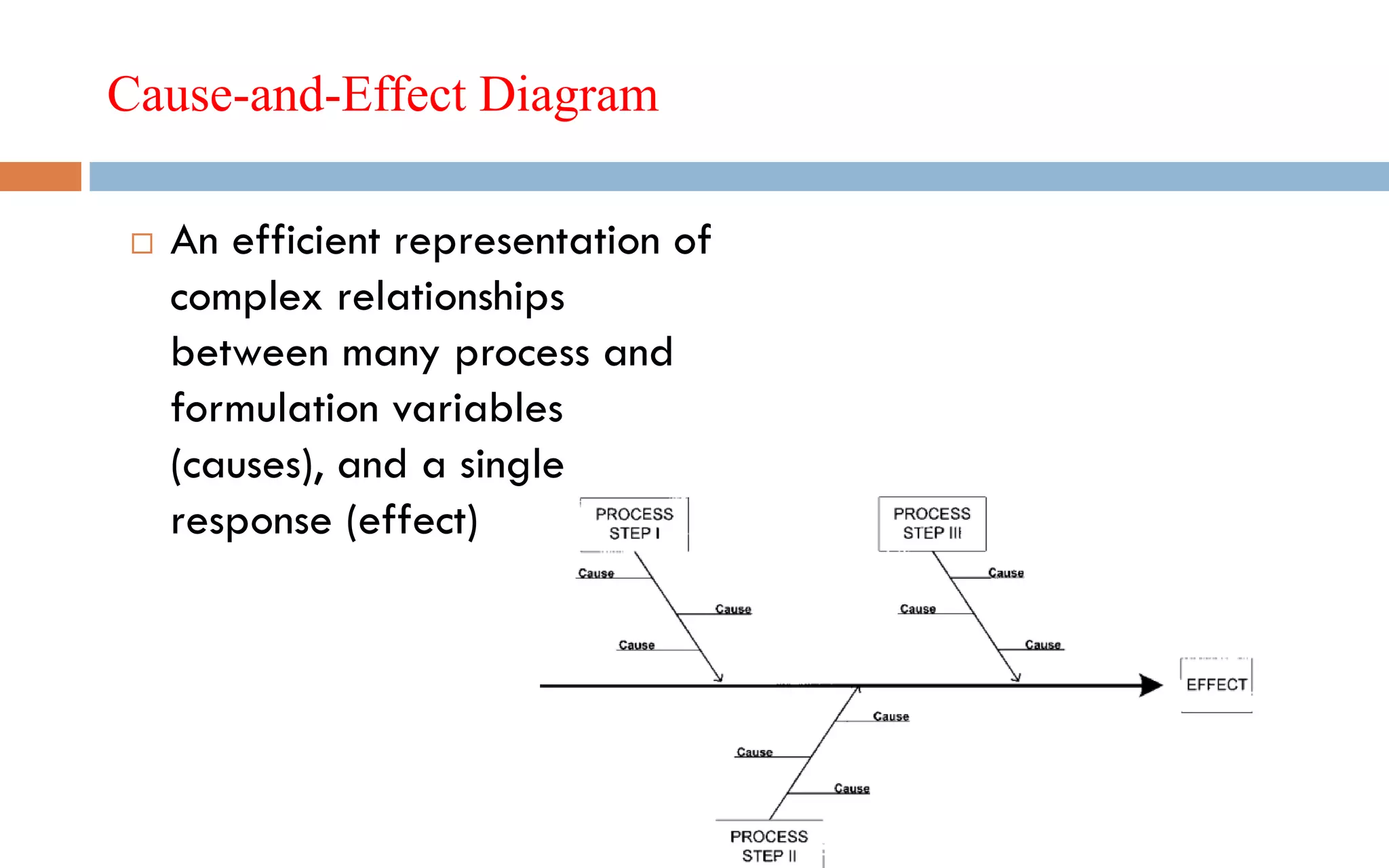
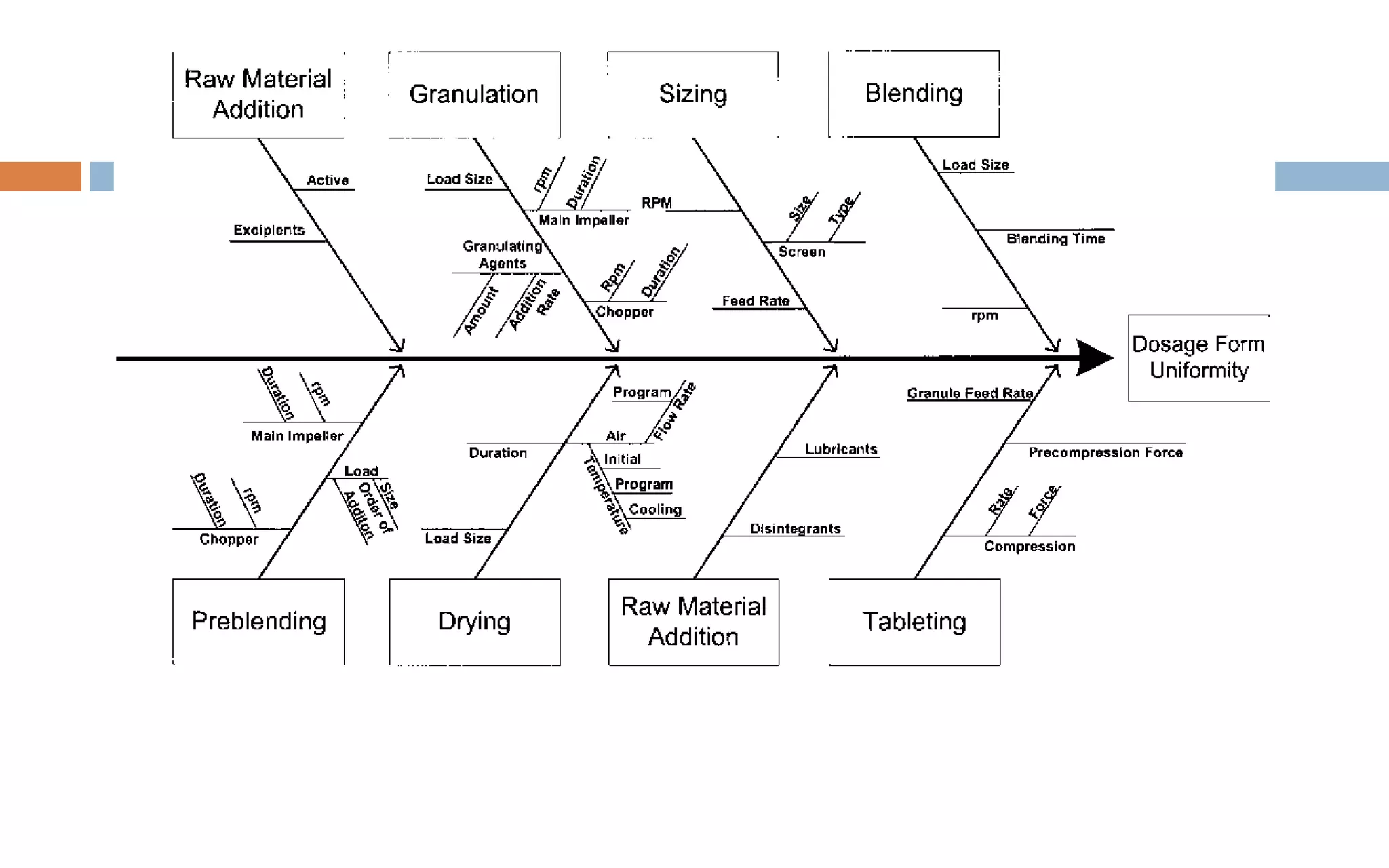
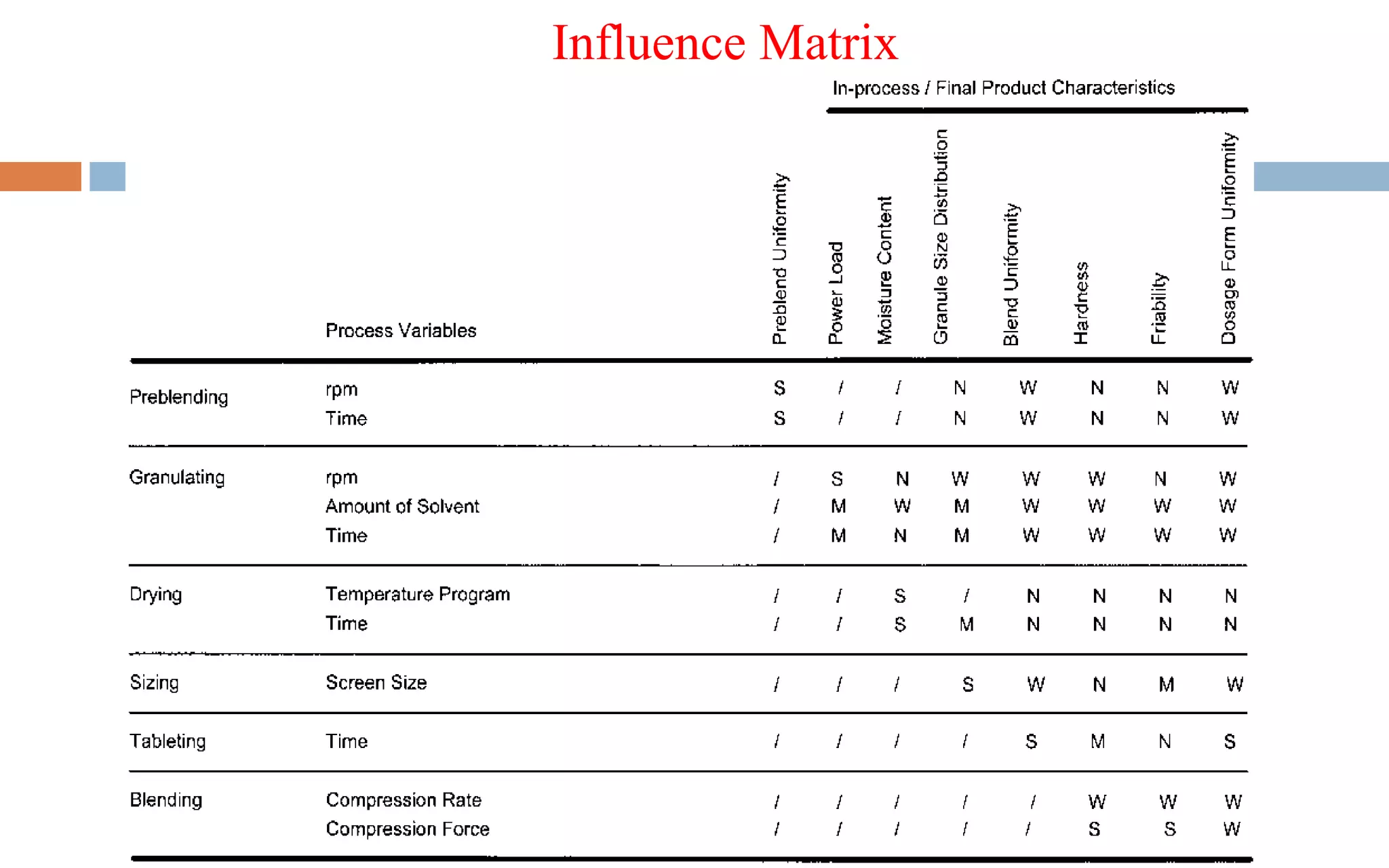
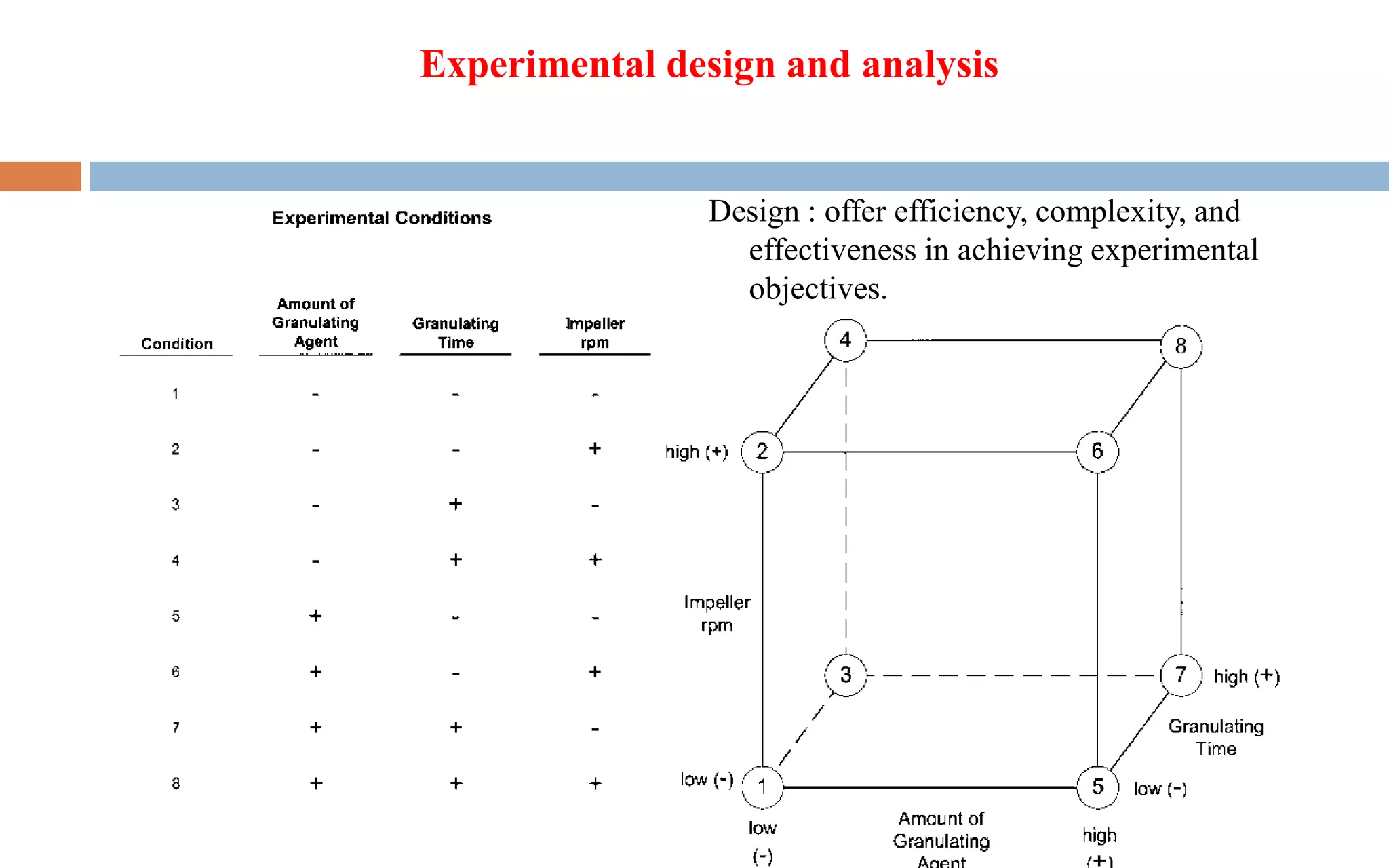
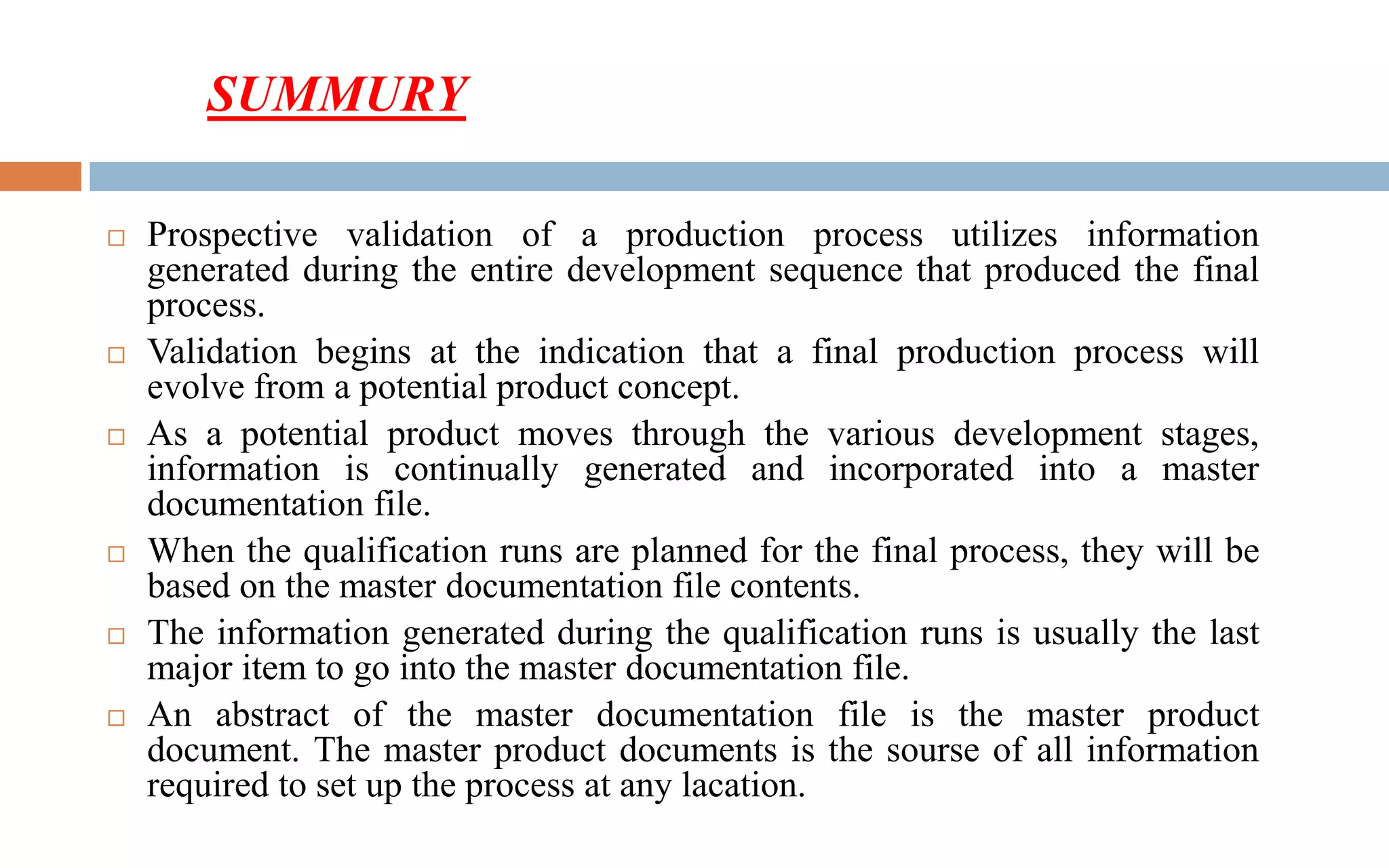
1) Prospective process validation requires a planned program from early development stages and utilizes information generated throughout development to validate the final production process.
2) Key aspects of the validation program include experimental design, documentation, defining objectives and variables, and maintaining effective organization and communication among team members.
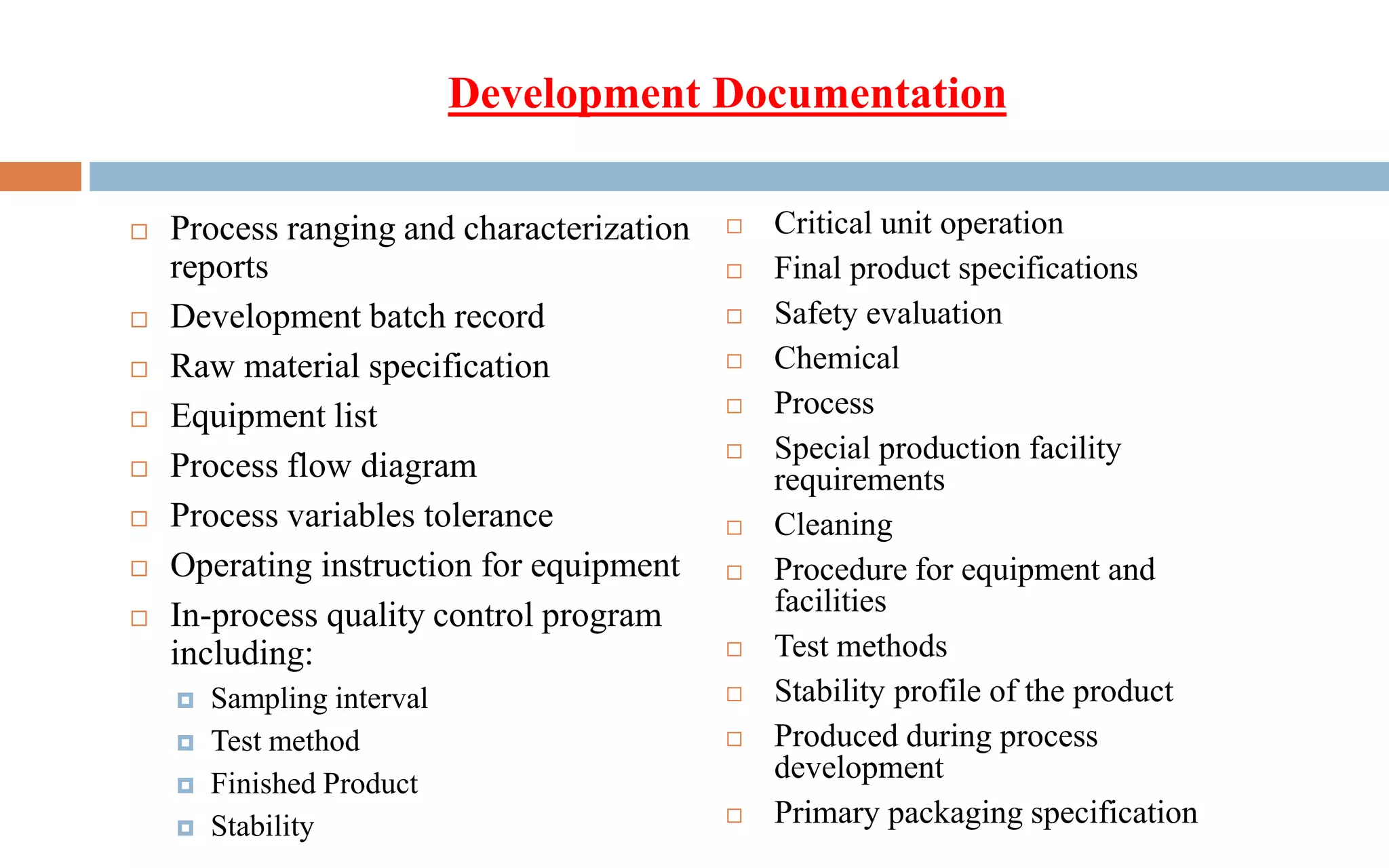
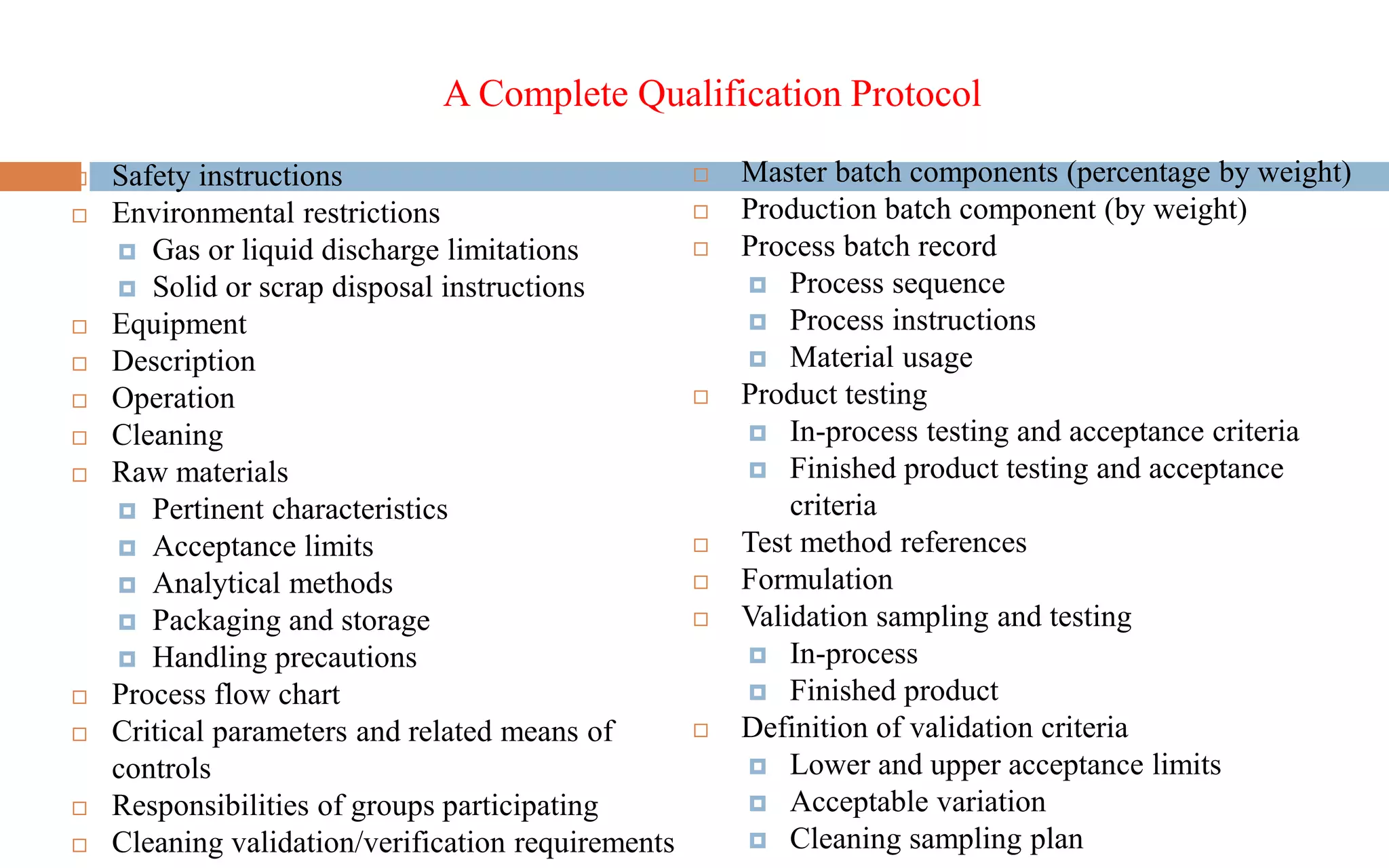
3) The validation is supported by a master documentation file containing all information needed to set up the validated production process.