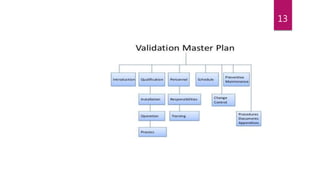
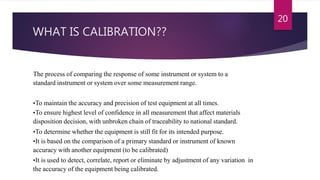
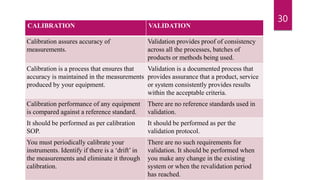
This document provides an overview of pharmaceutical validation and calibration processes. It discusses the objectives of validation which include reducing regulatory risks and defects. The scope of validation covers analytical, facilities, manufacturing, product design, cleaning, instrumentation, utilities, materials and equipment. A validation master plan outlines the validation strategy and includes qualification methods, personnel responsibilities, schedules, documentation and change control. Similarly, a calibration master plan ensures equipment is routinely calibrated against reference standards to ensure proper performance and measurement traceability.