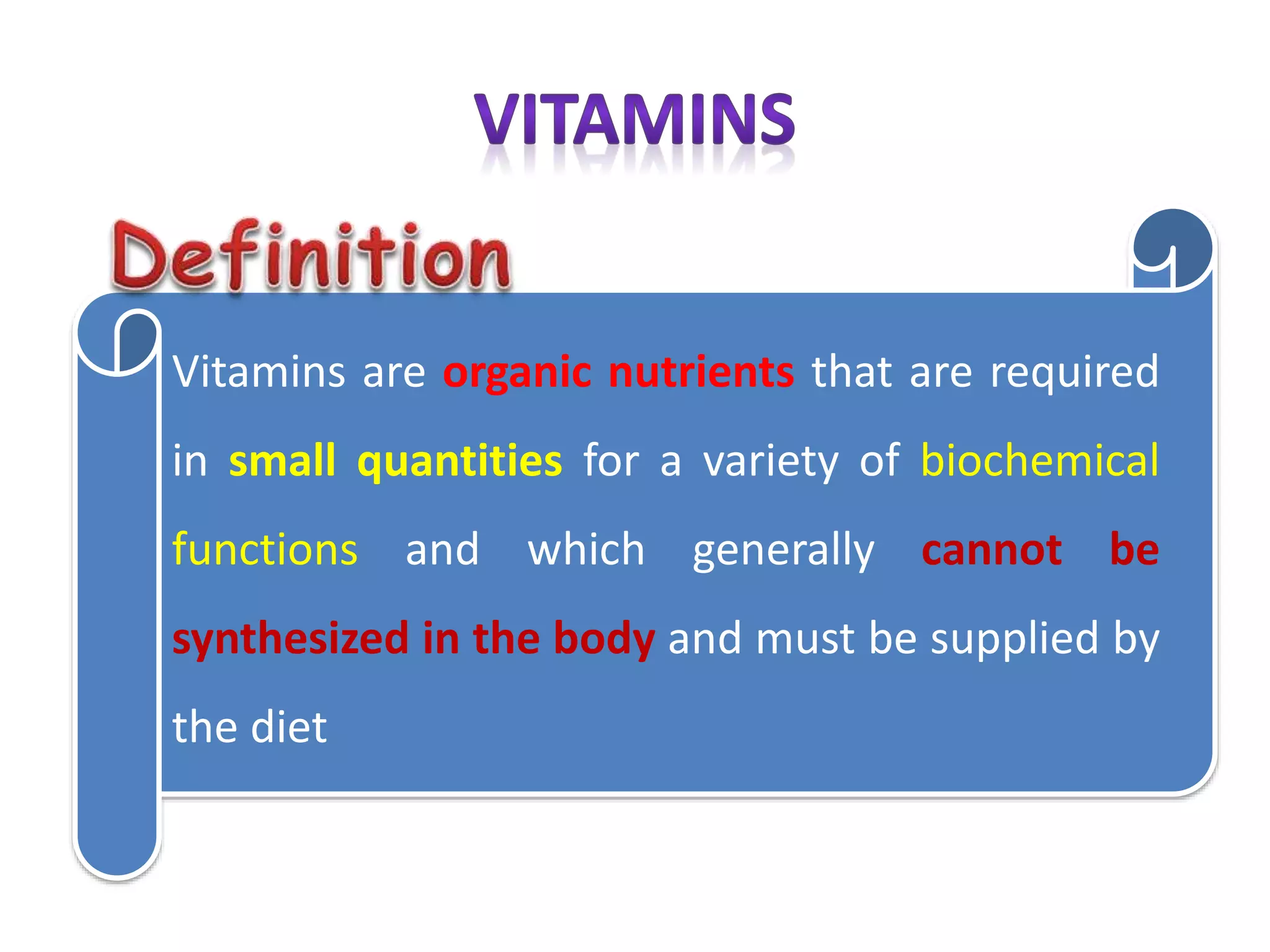
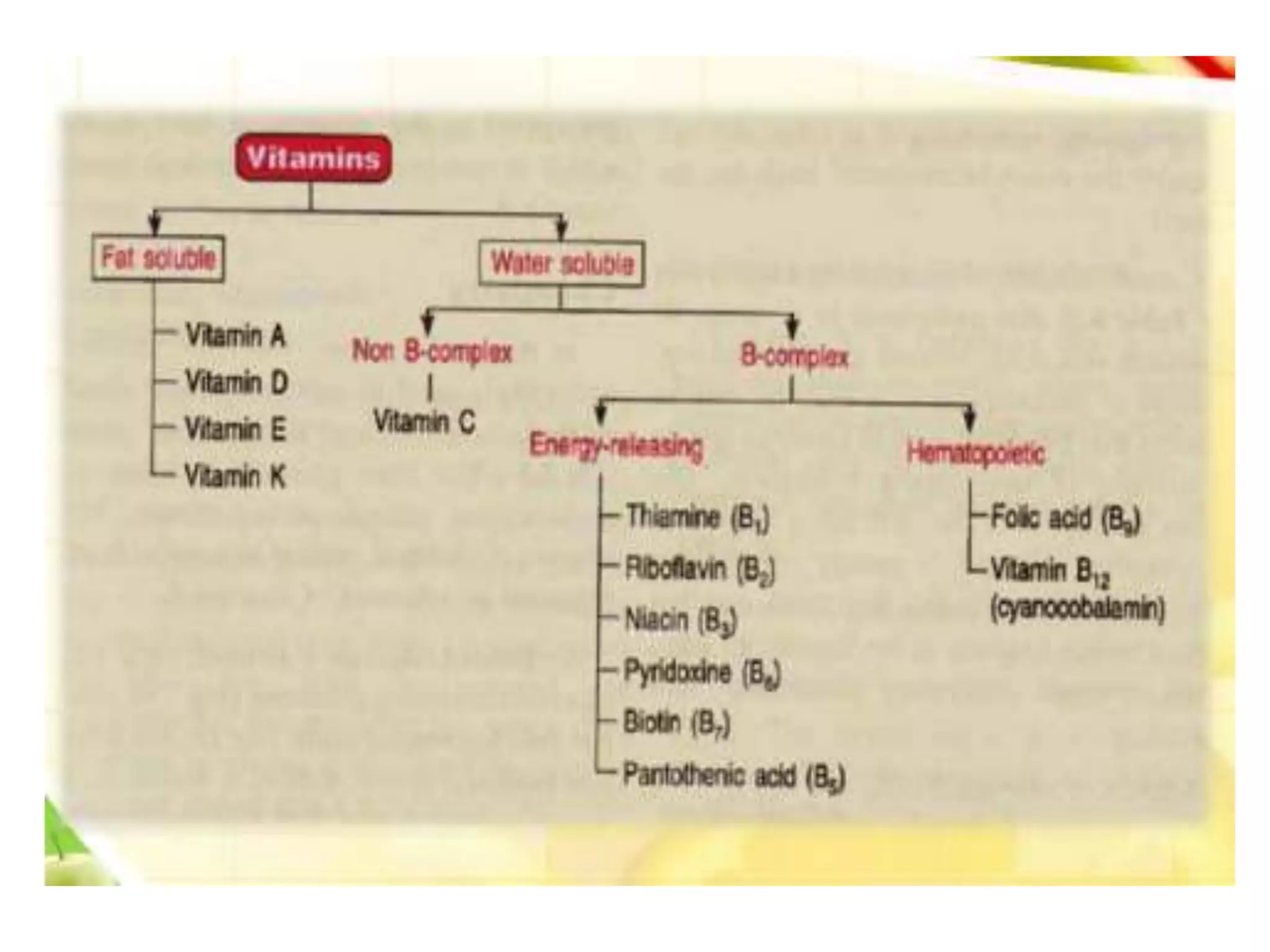
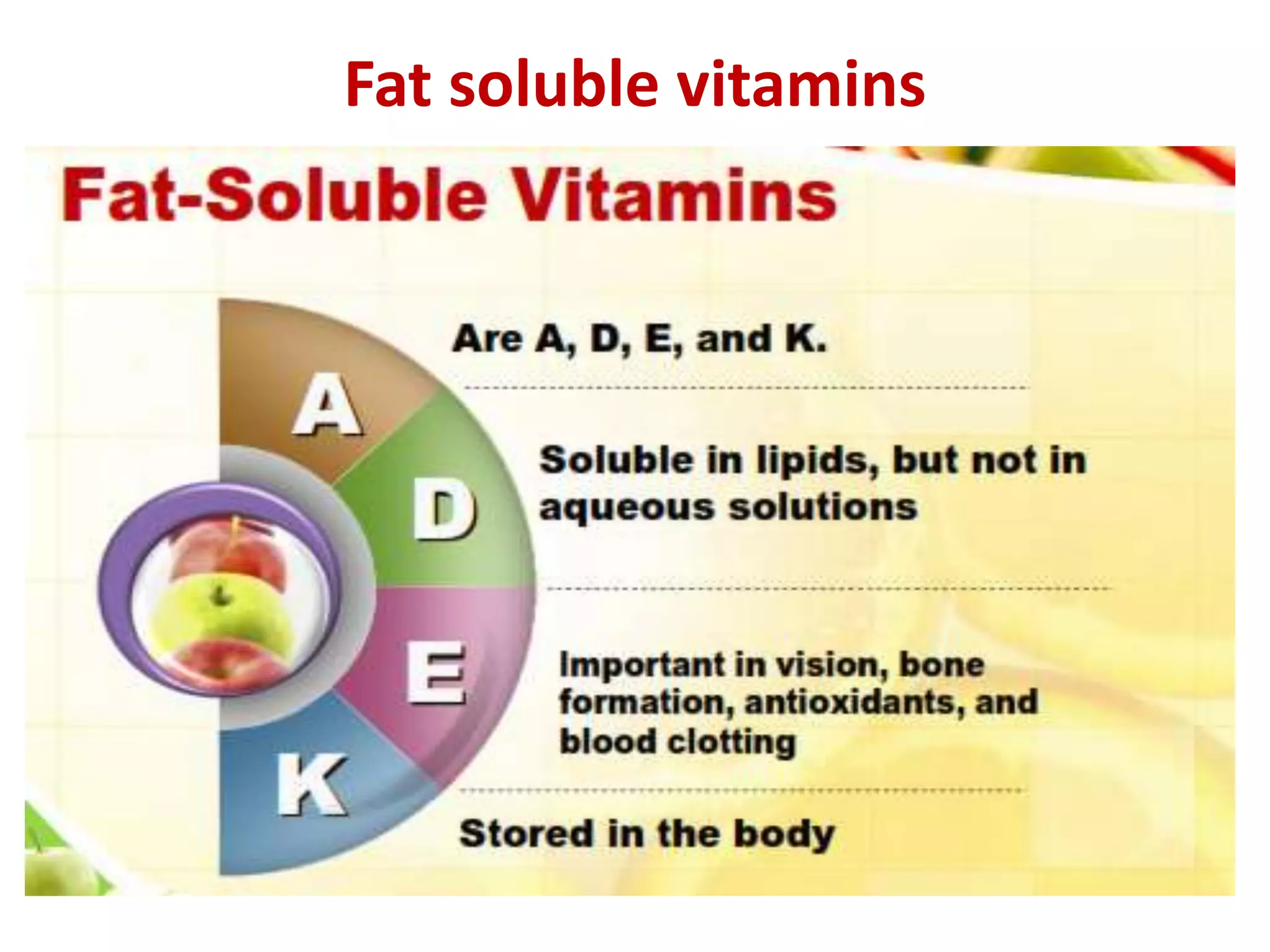
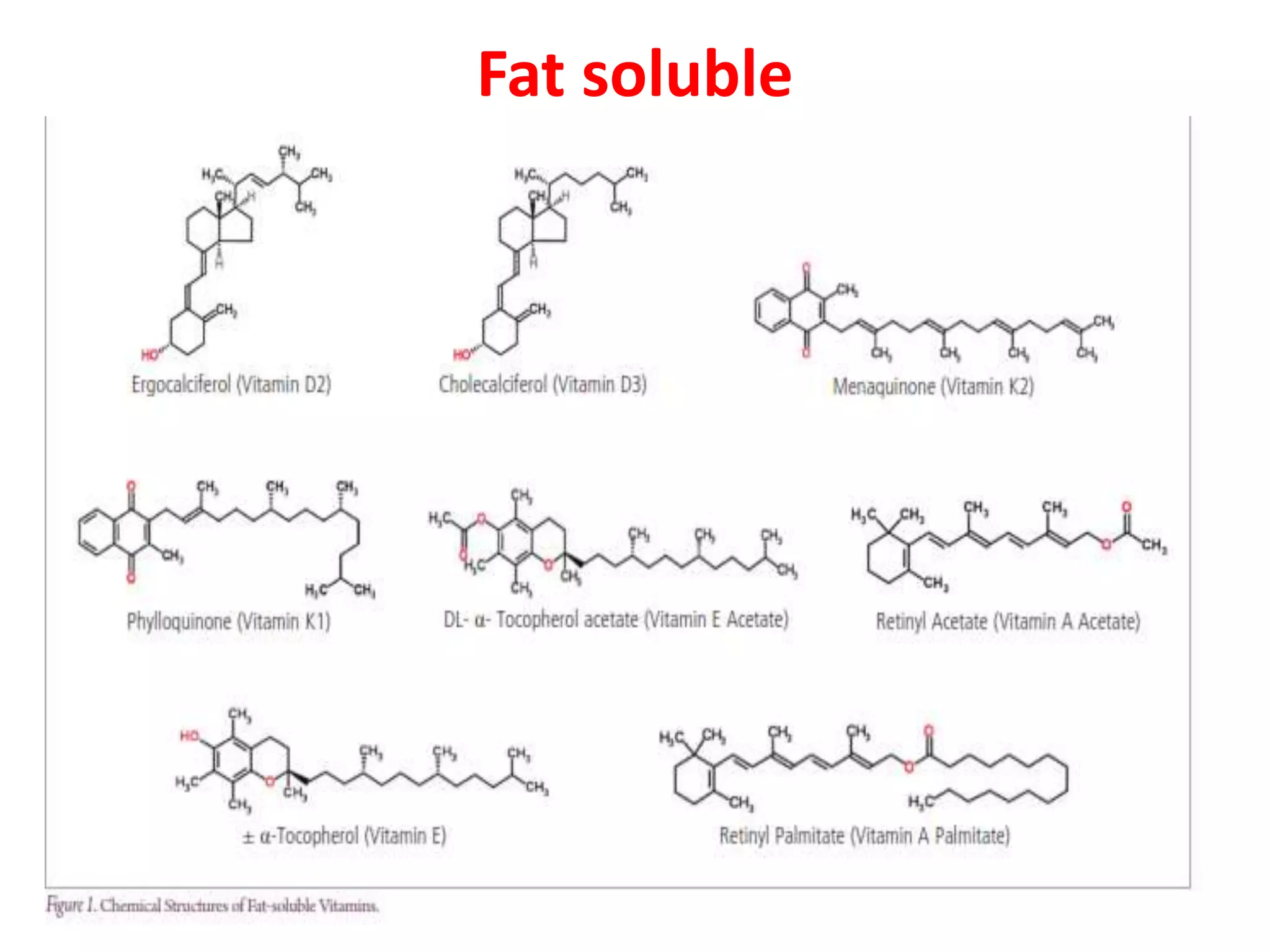
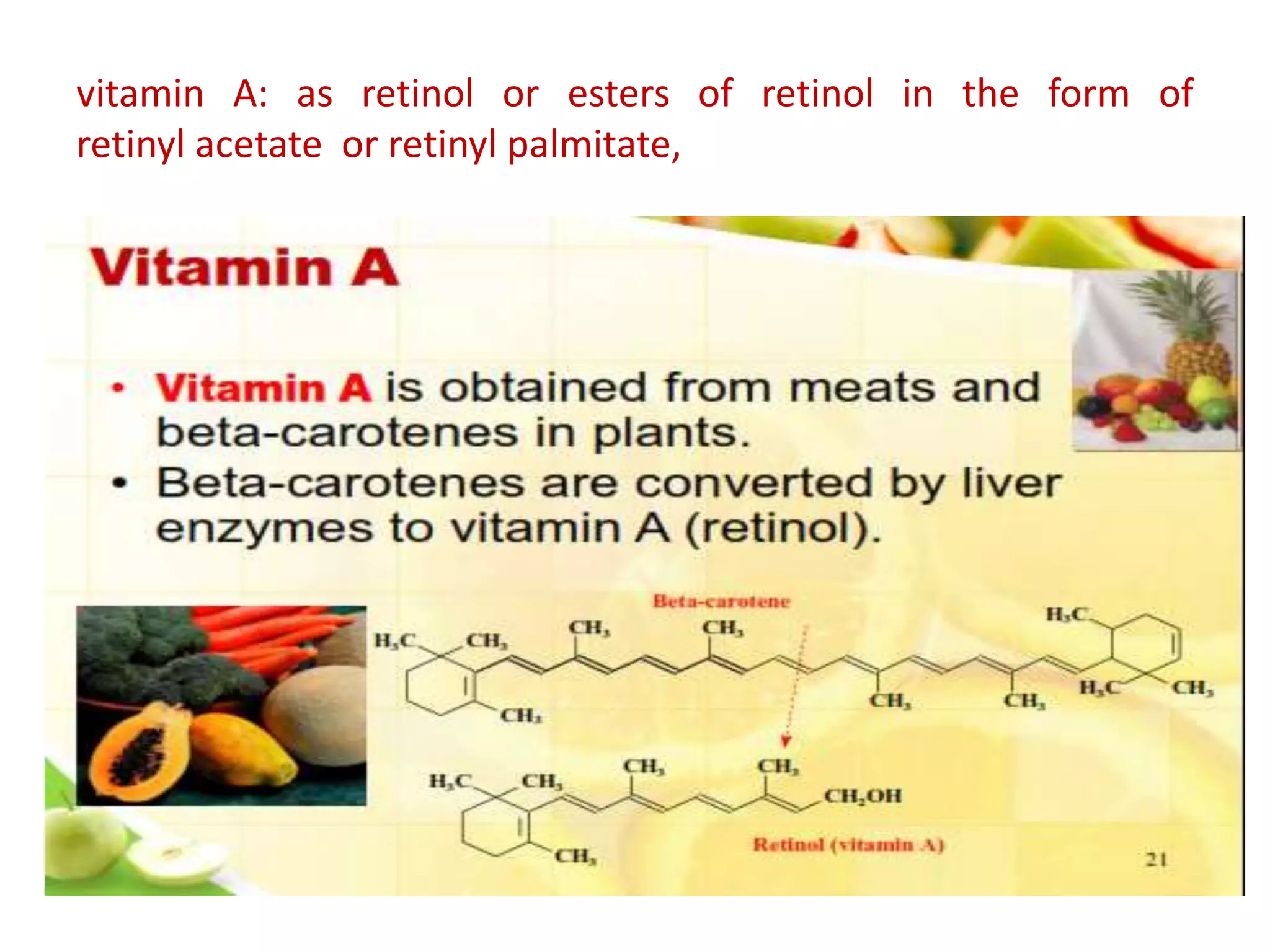
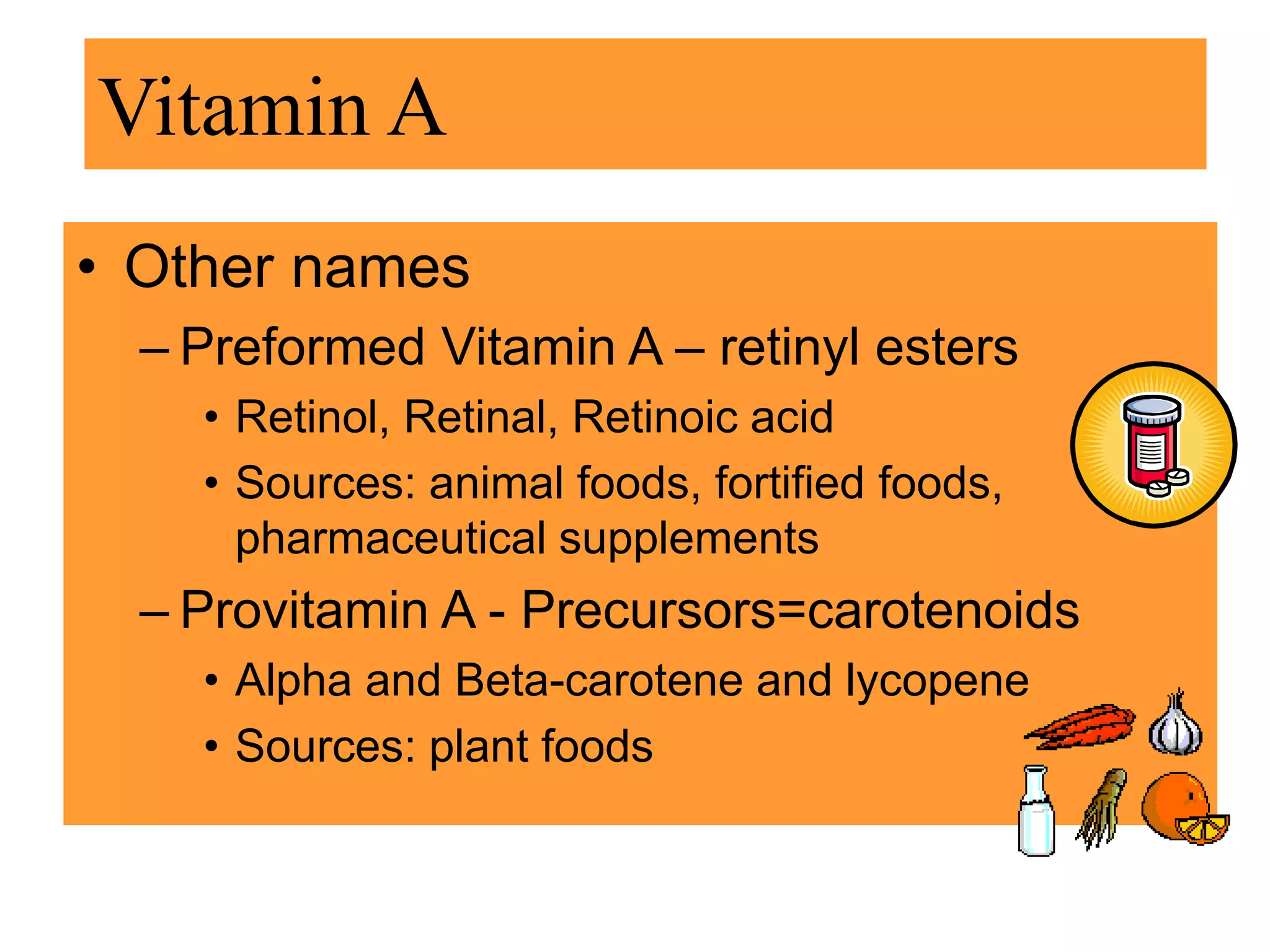
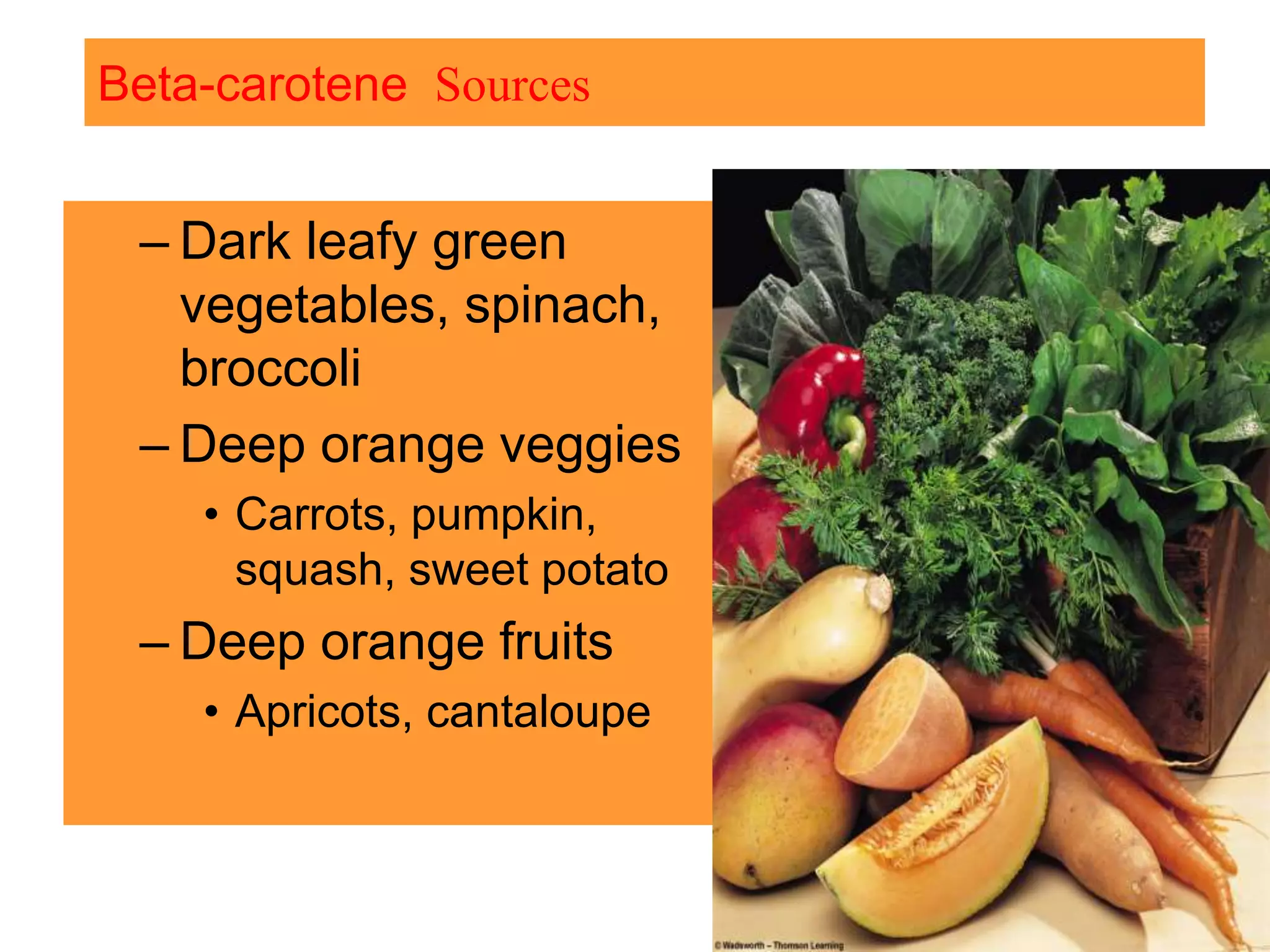
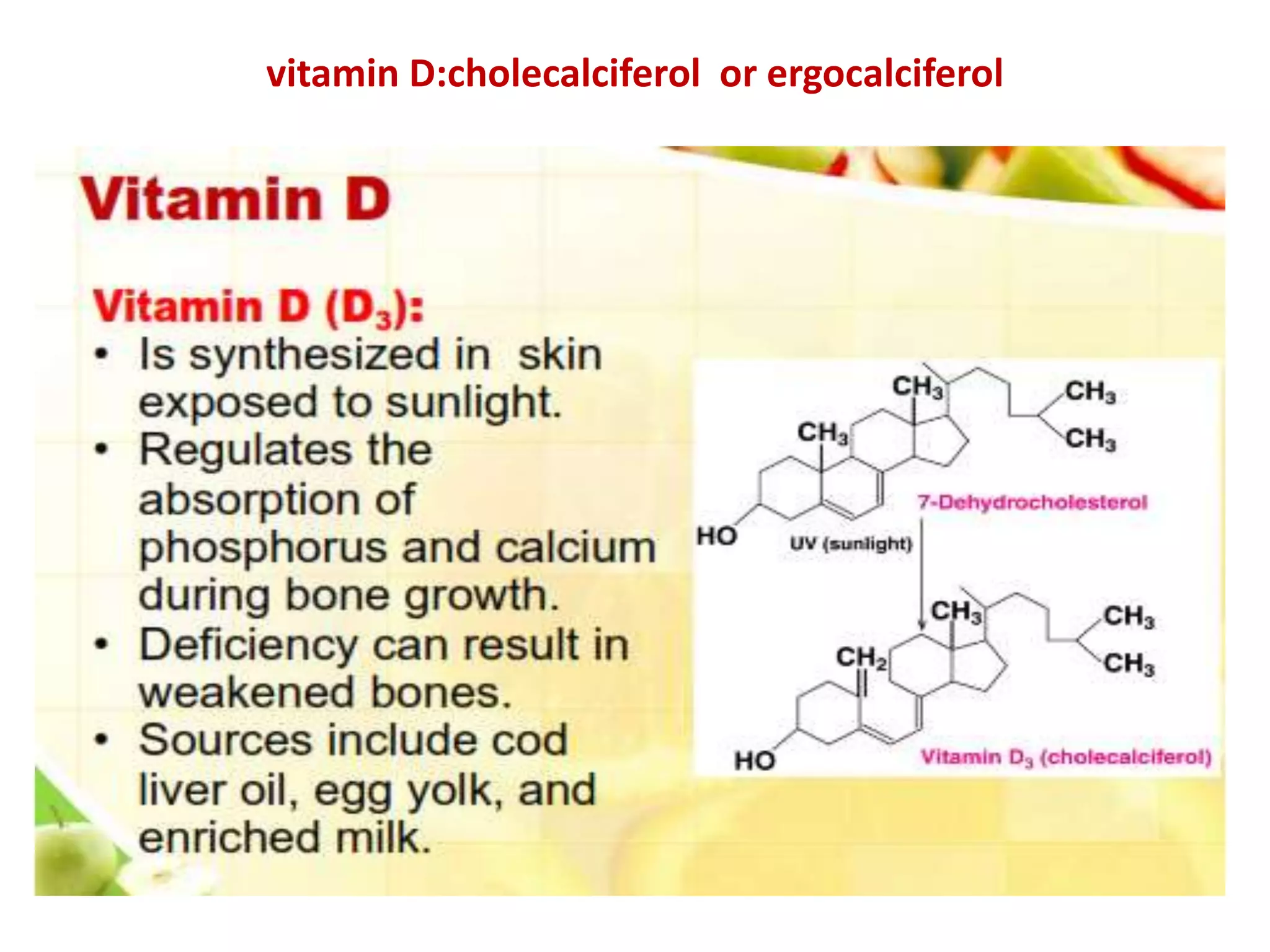
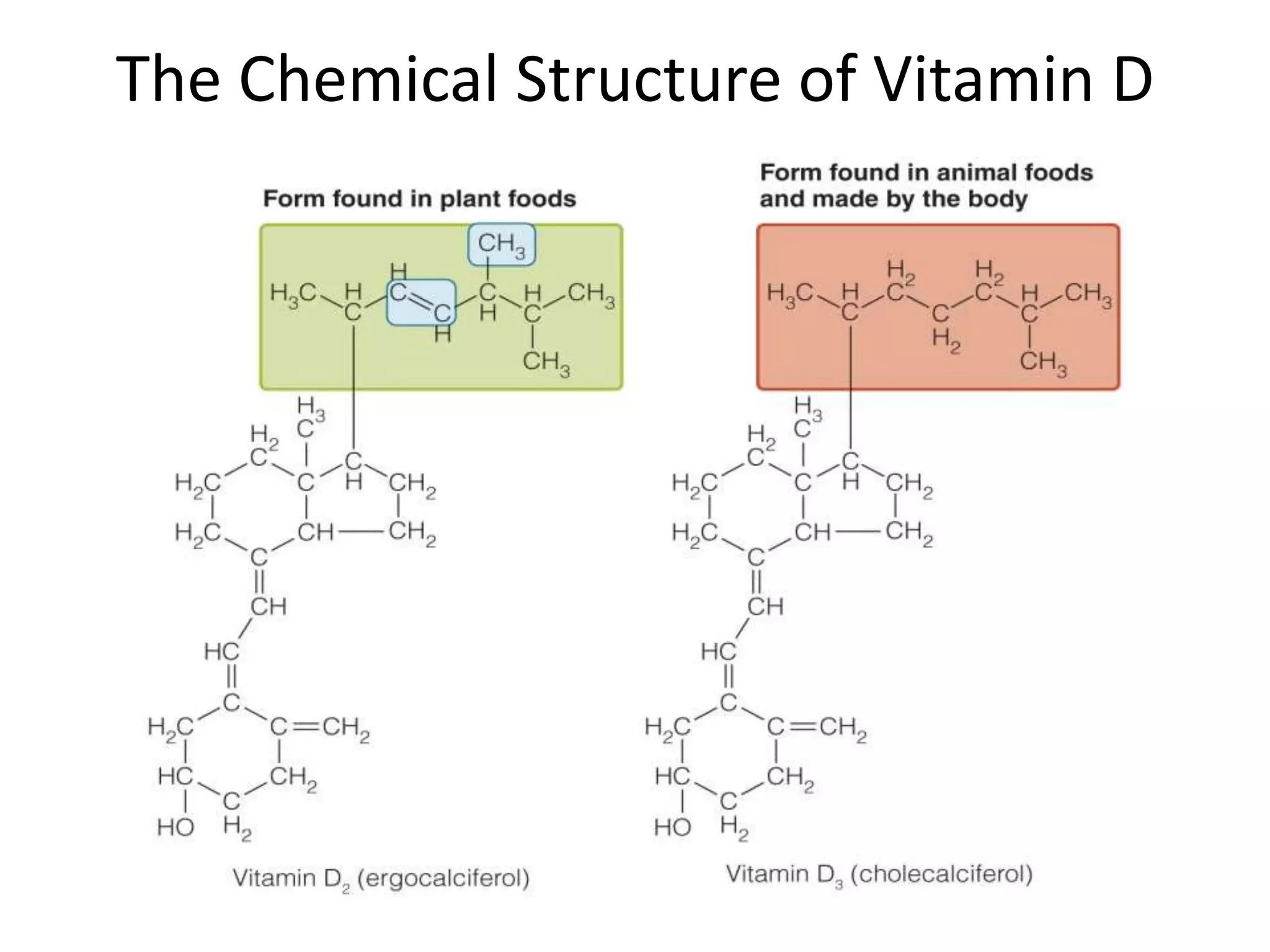
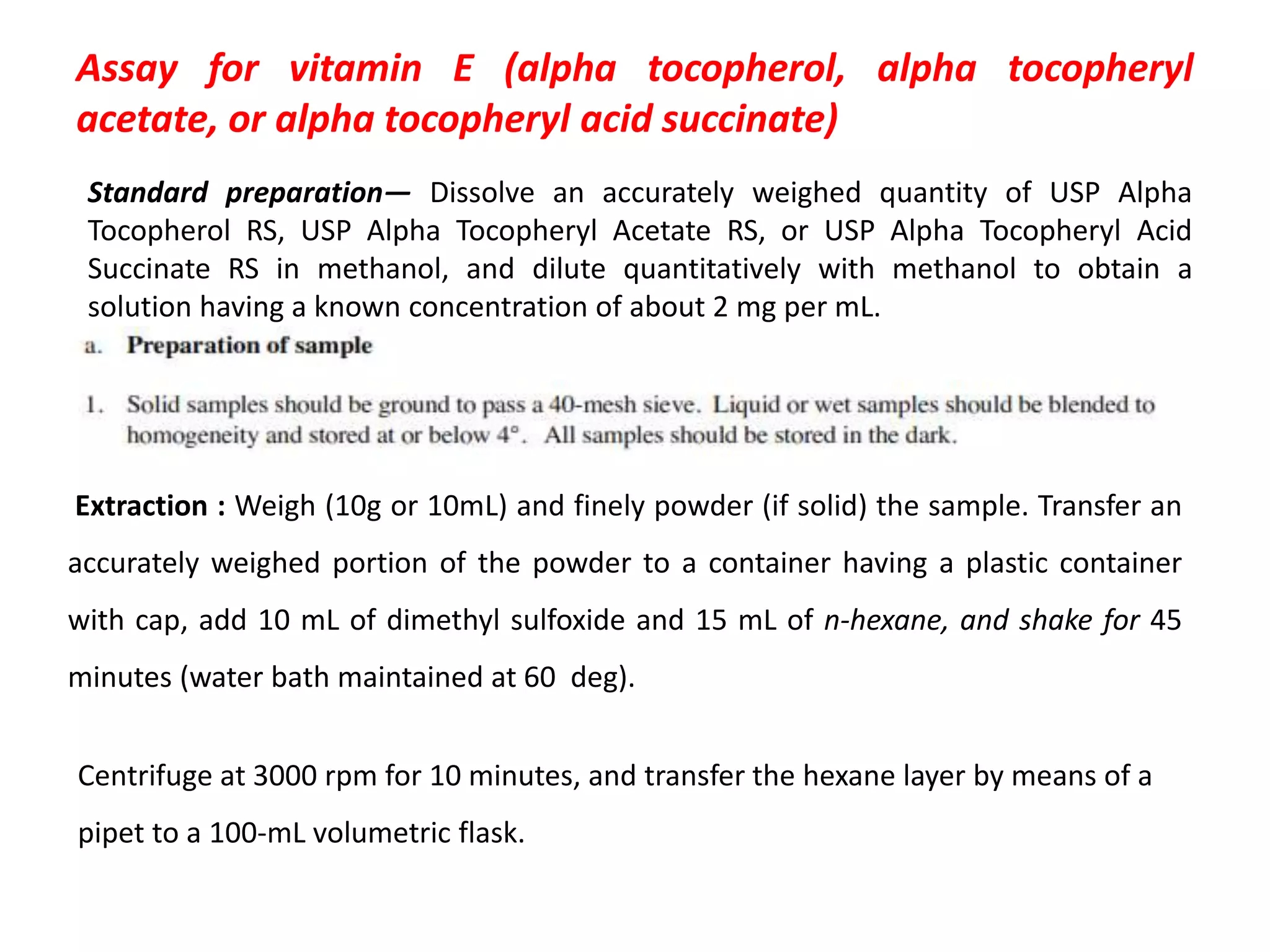
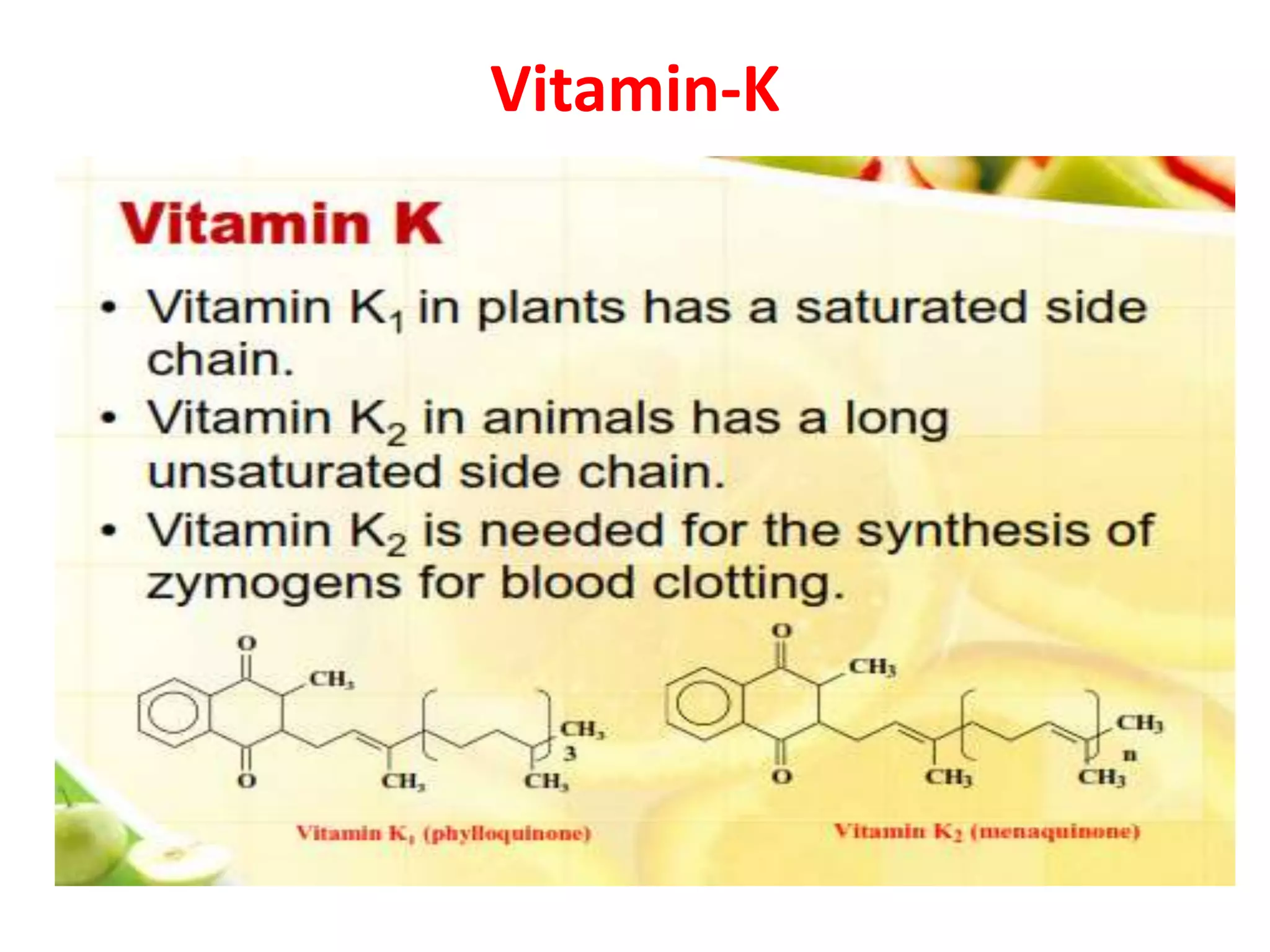
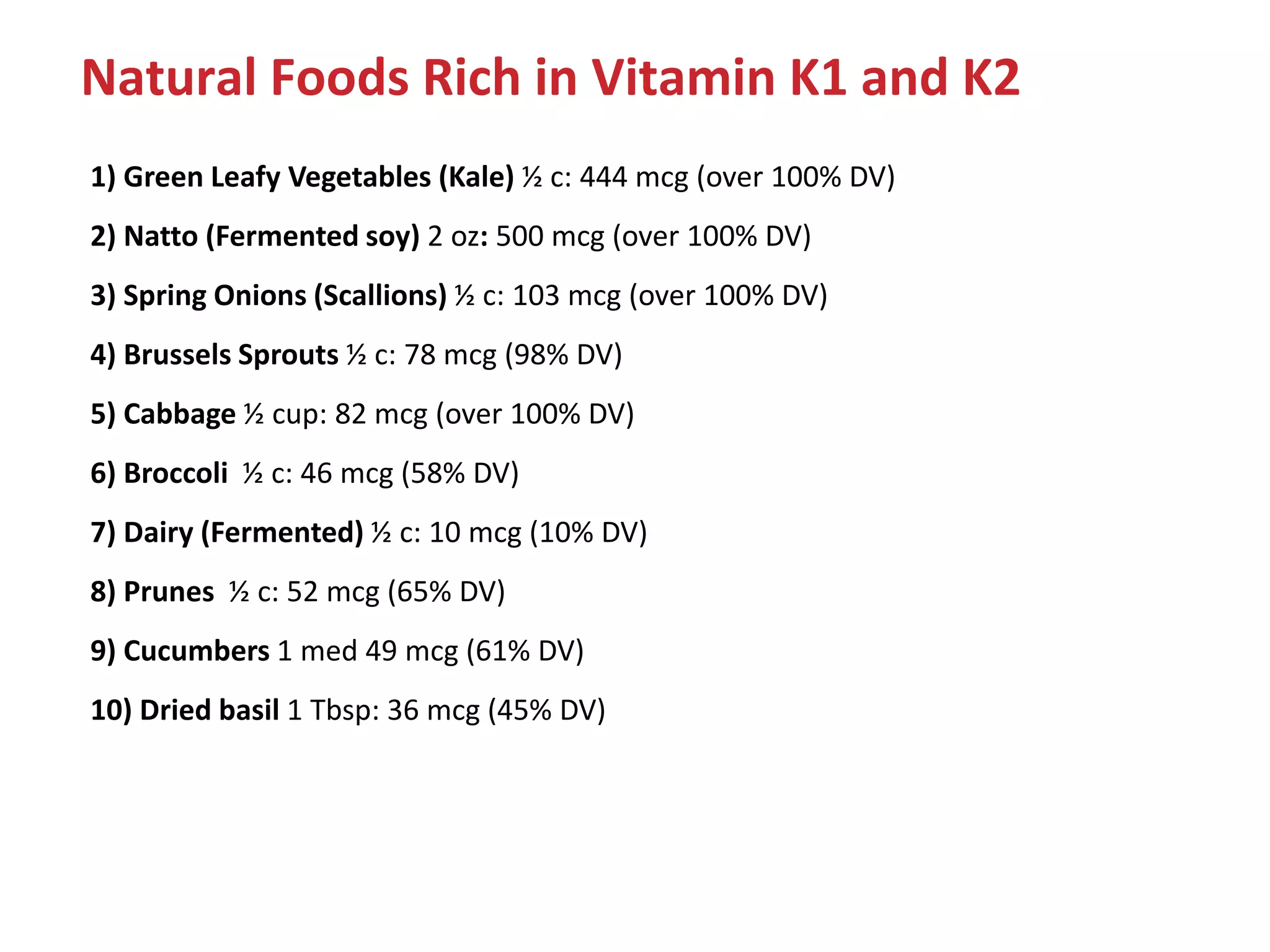
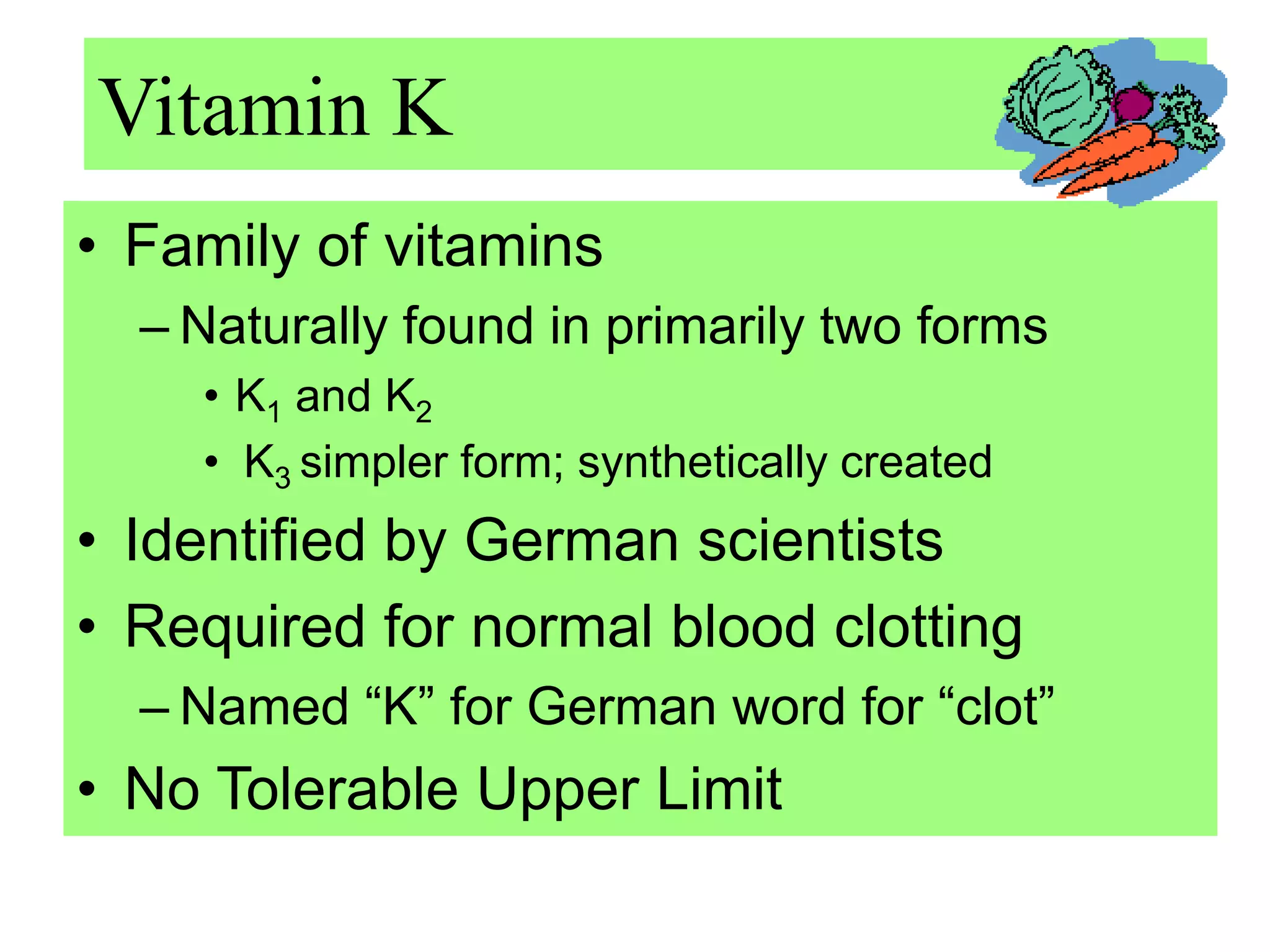
Vitamin K plays an essential role in blood clotting and bone health. It exists in two primary forms, K1 and K2, with K2 having greater potency and a wider range of functions. Key sources of vitamin K include green leafy vegetables, fermented foods like natto, and bacteria in the intestines. Adequate vitamin K is required for blood clotting by enabling the liver to synthesize clotting proteins, as well as supporting bone mineralization and vascular health.