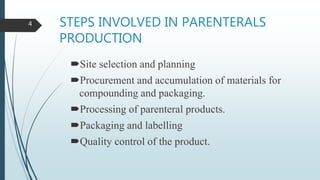
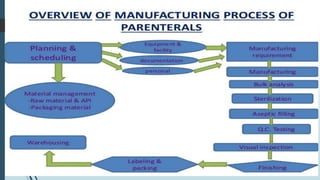
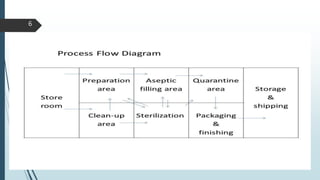
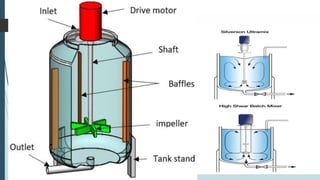
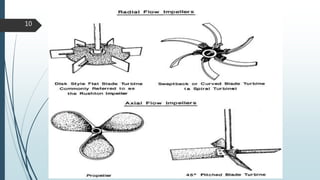
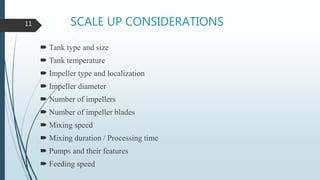
This document discusses the scale-up considerations for producing parenteral drugs on a pilot plant scale. It describes the key unit operations in parenteral production as mixing, sterilization, filtration, filling and sealing. For each unit operation, parameters that must be considered for scale-up are identified, such as tank size and type, impeller design, membrane size, filling rate and container size. Maintaining sterility and avoiding issues like precipitation or clogging are important challenges addressed during scale-up. Quality control tests are used to evaluate the scaled processes. Proper scale-up allows efficient transition from laboratory to commercial production of injectable drug products.