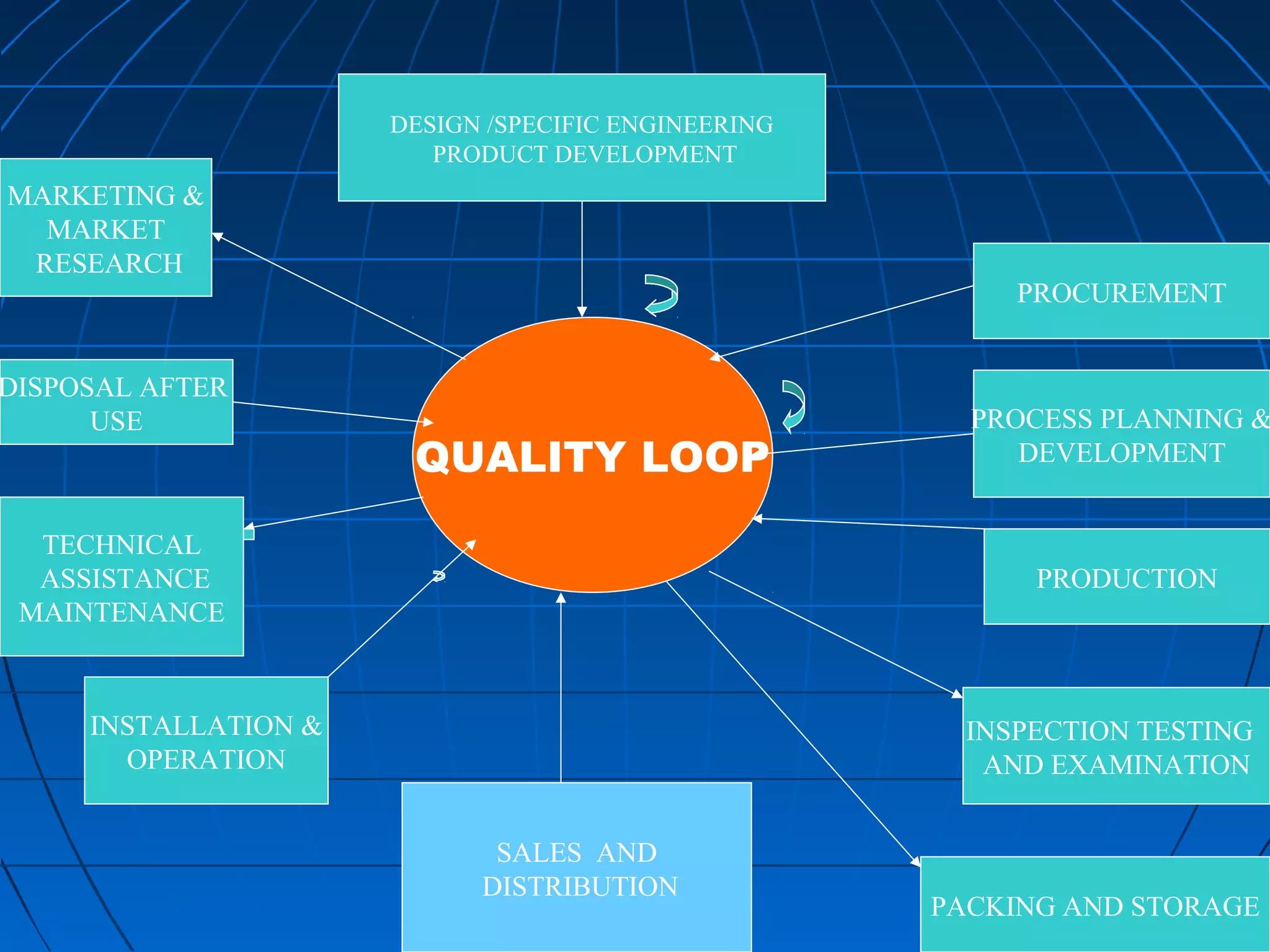
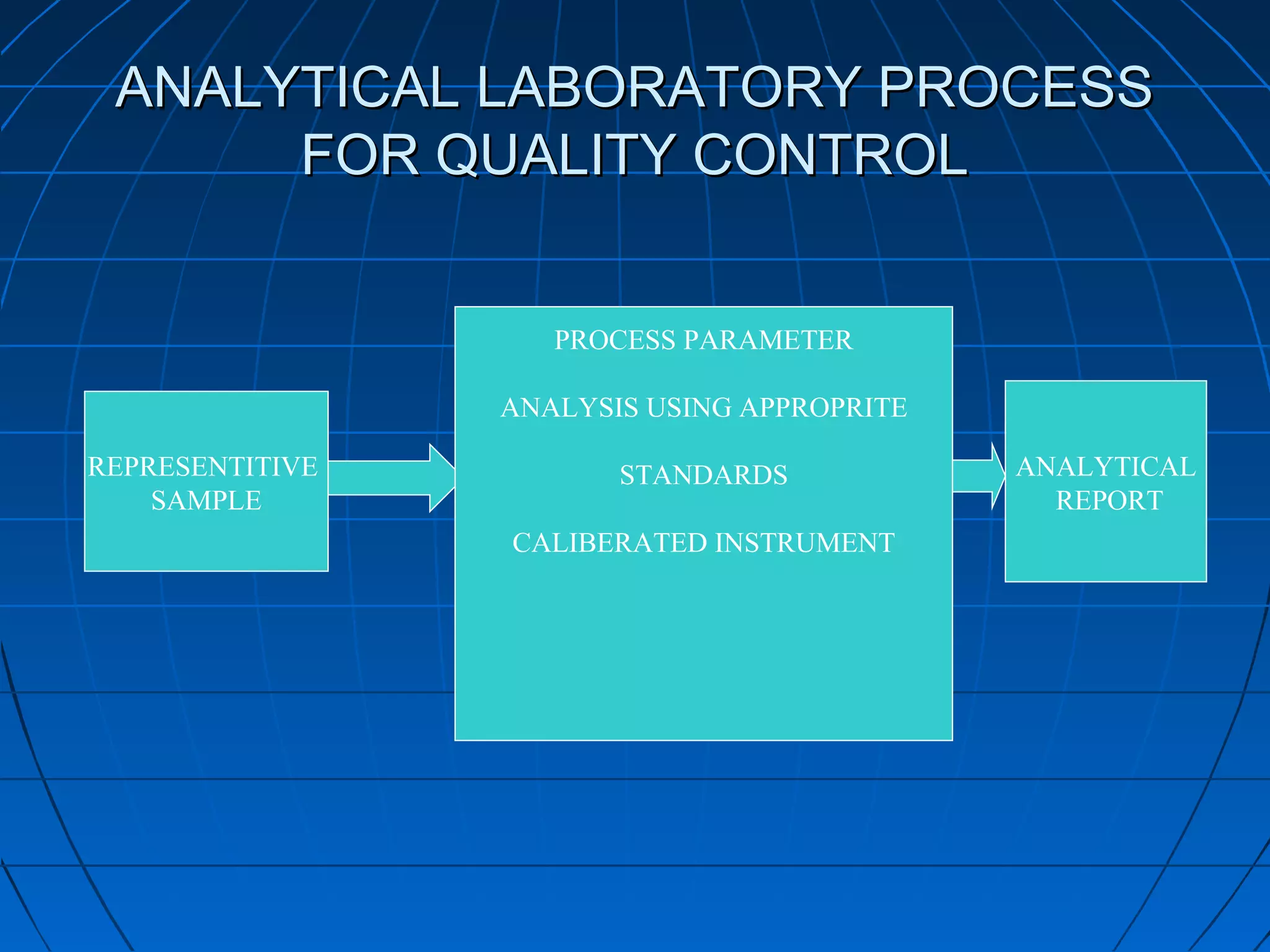
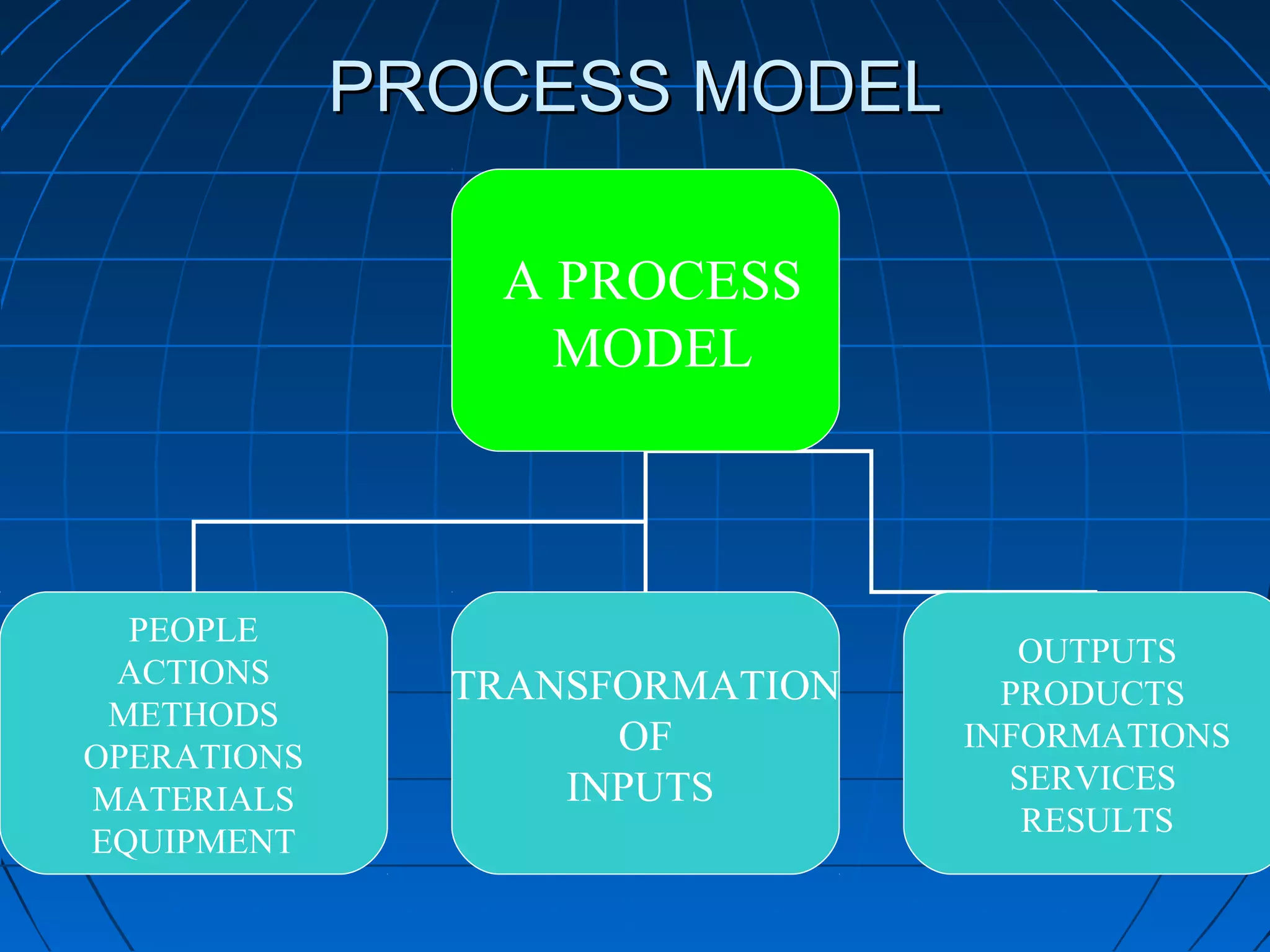
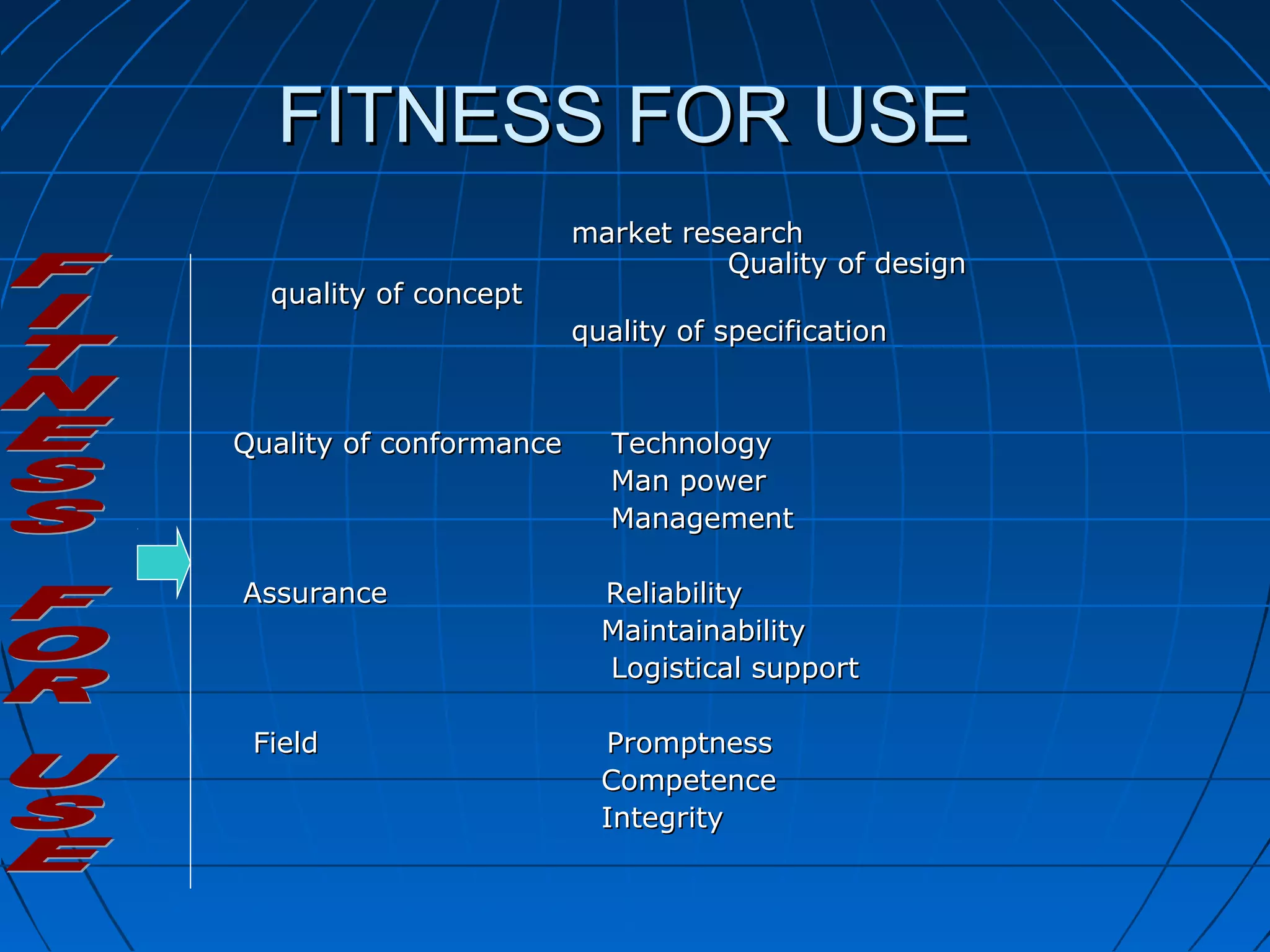
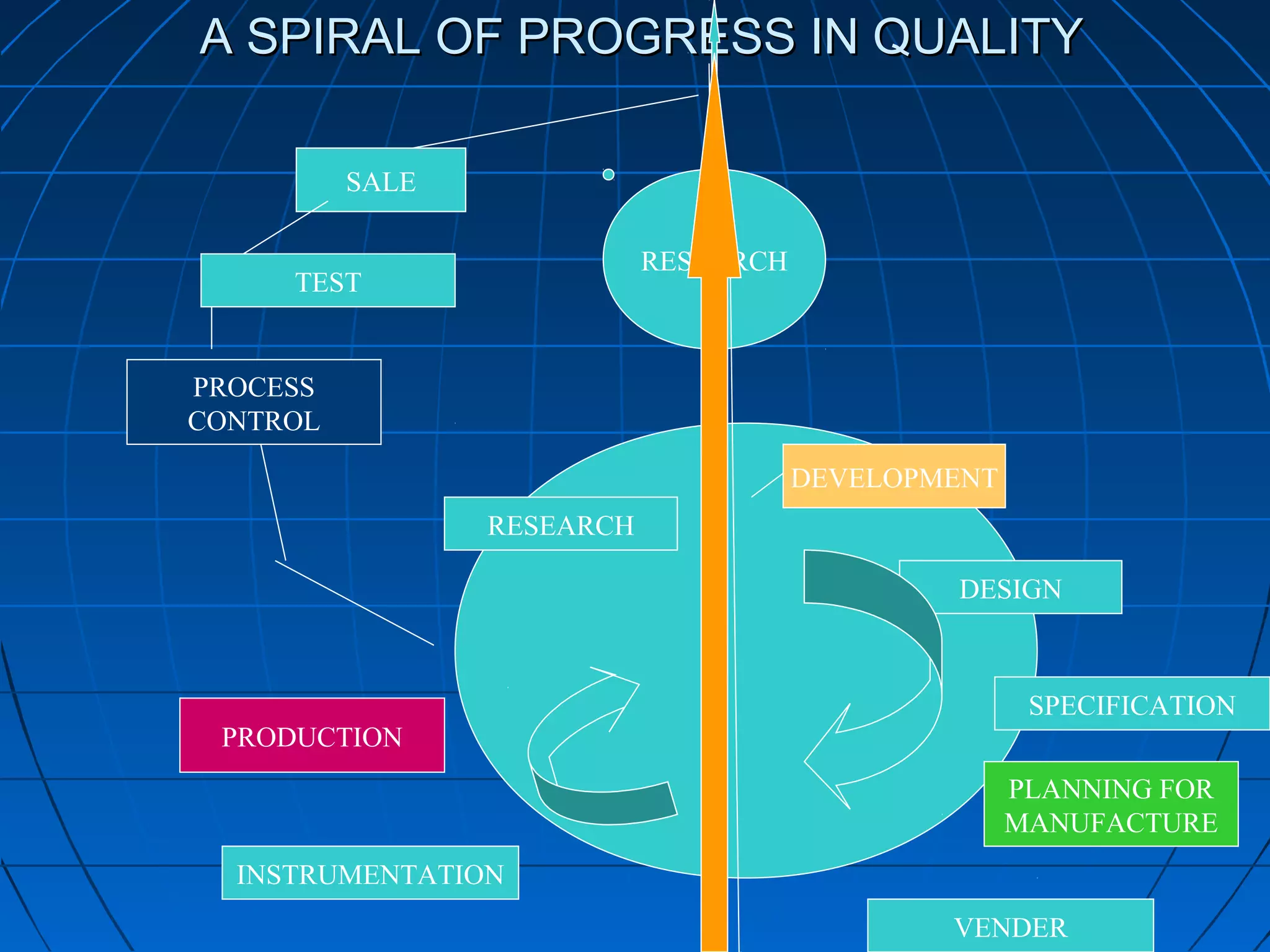
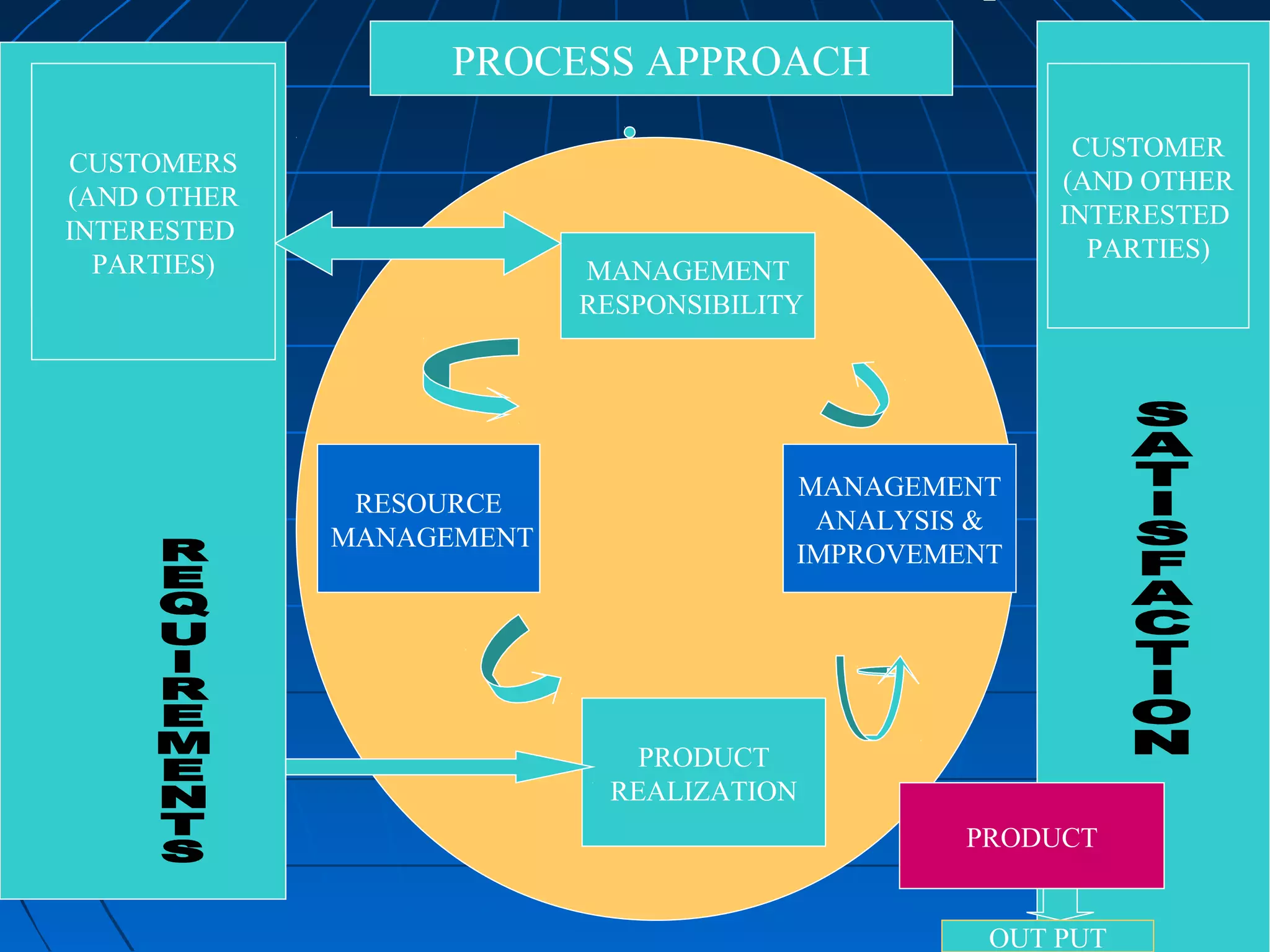
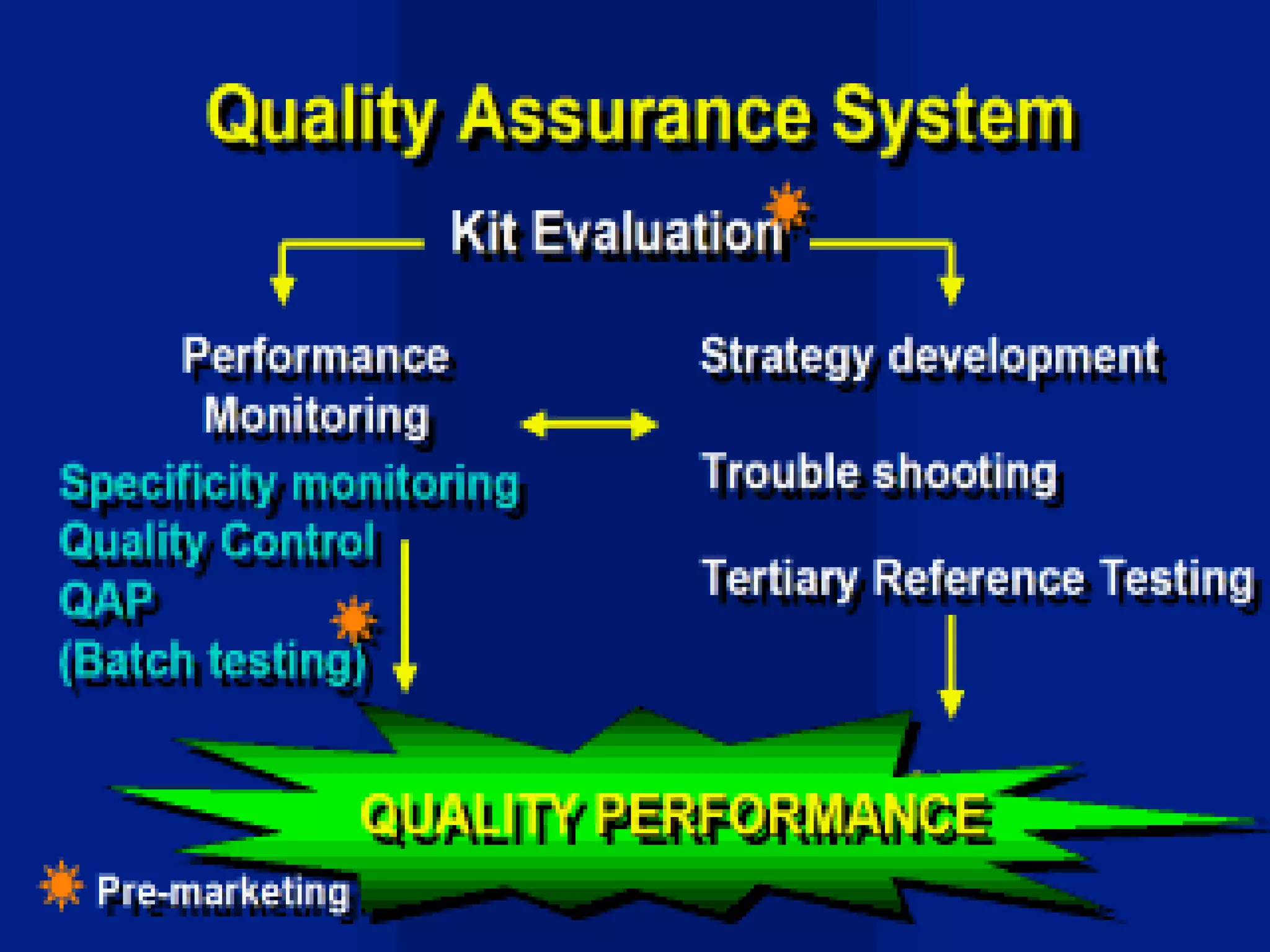
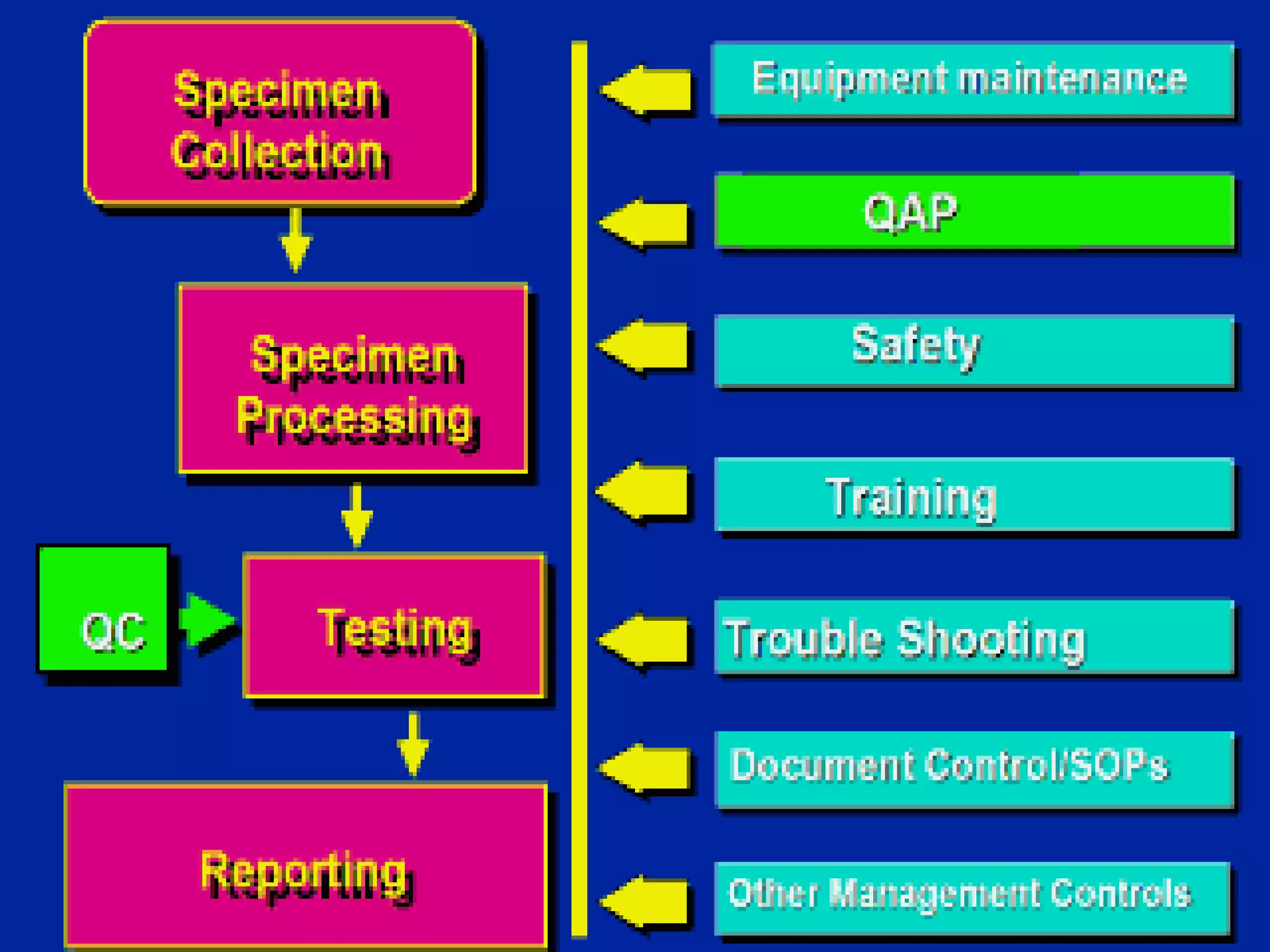
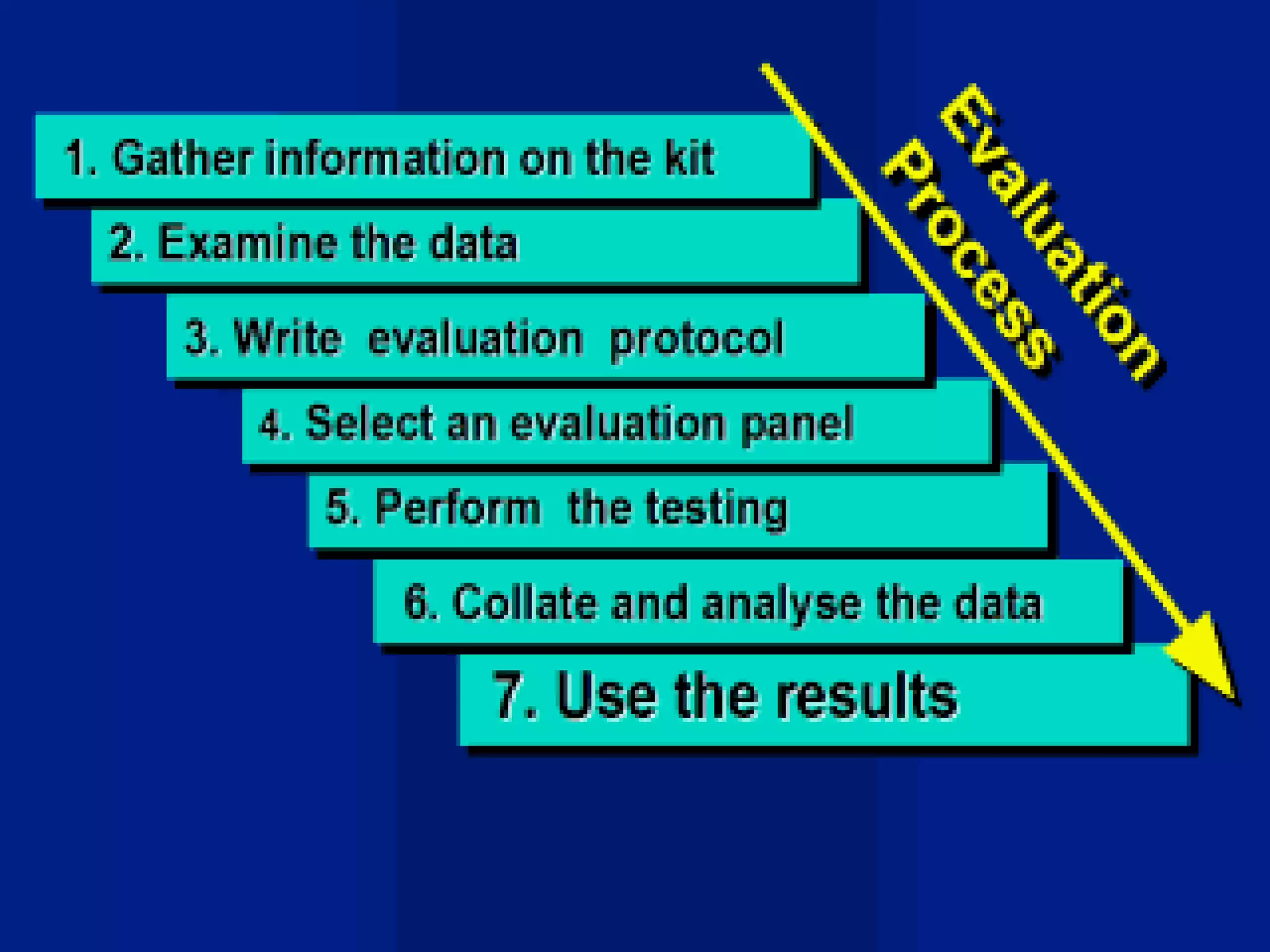
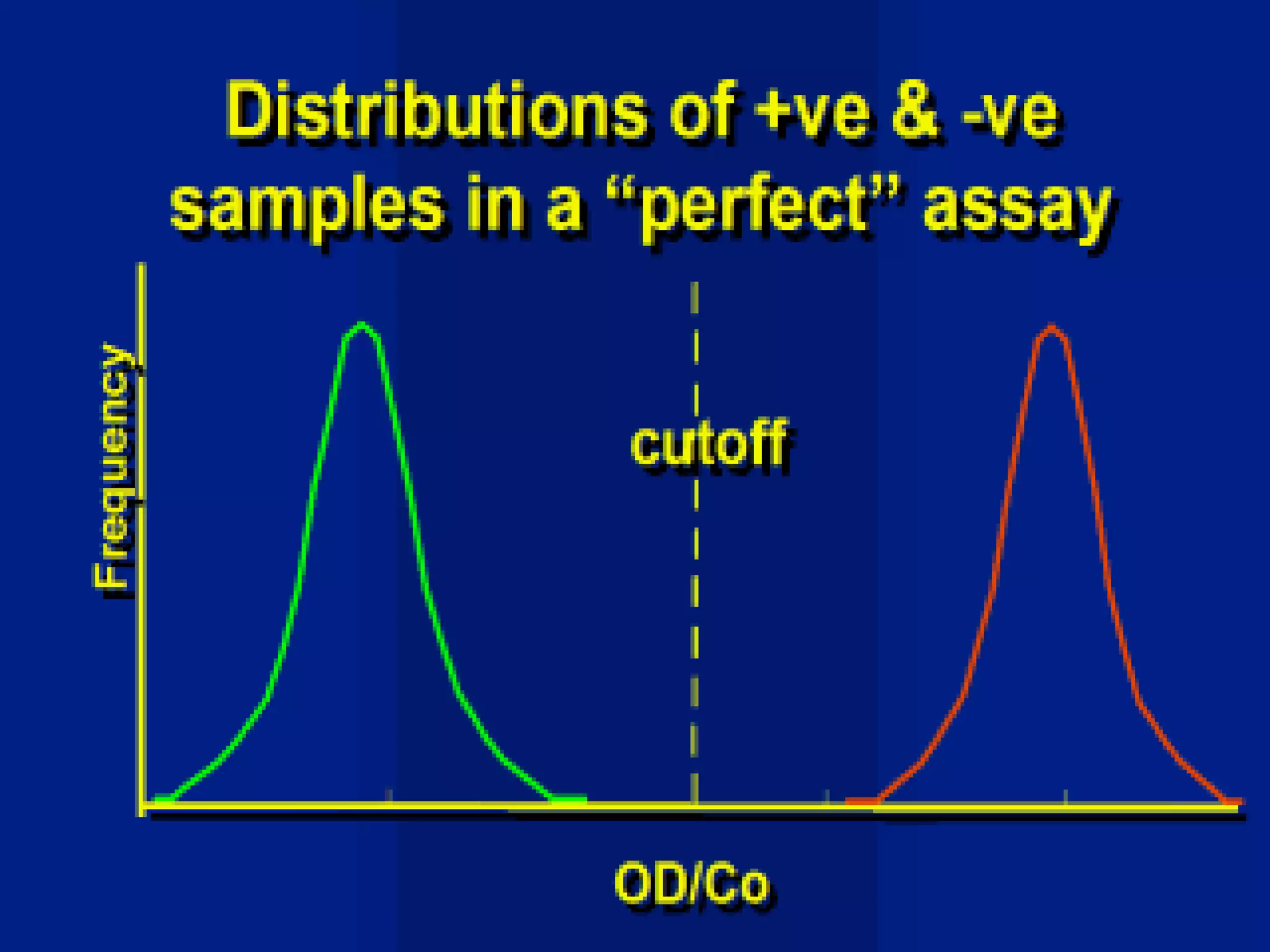
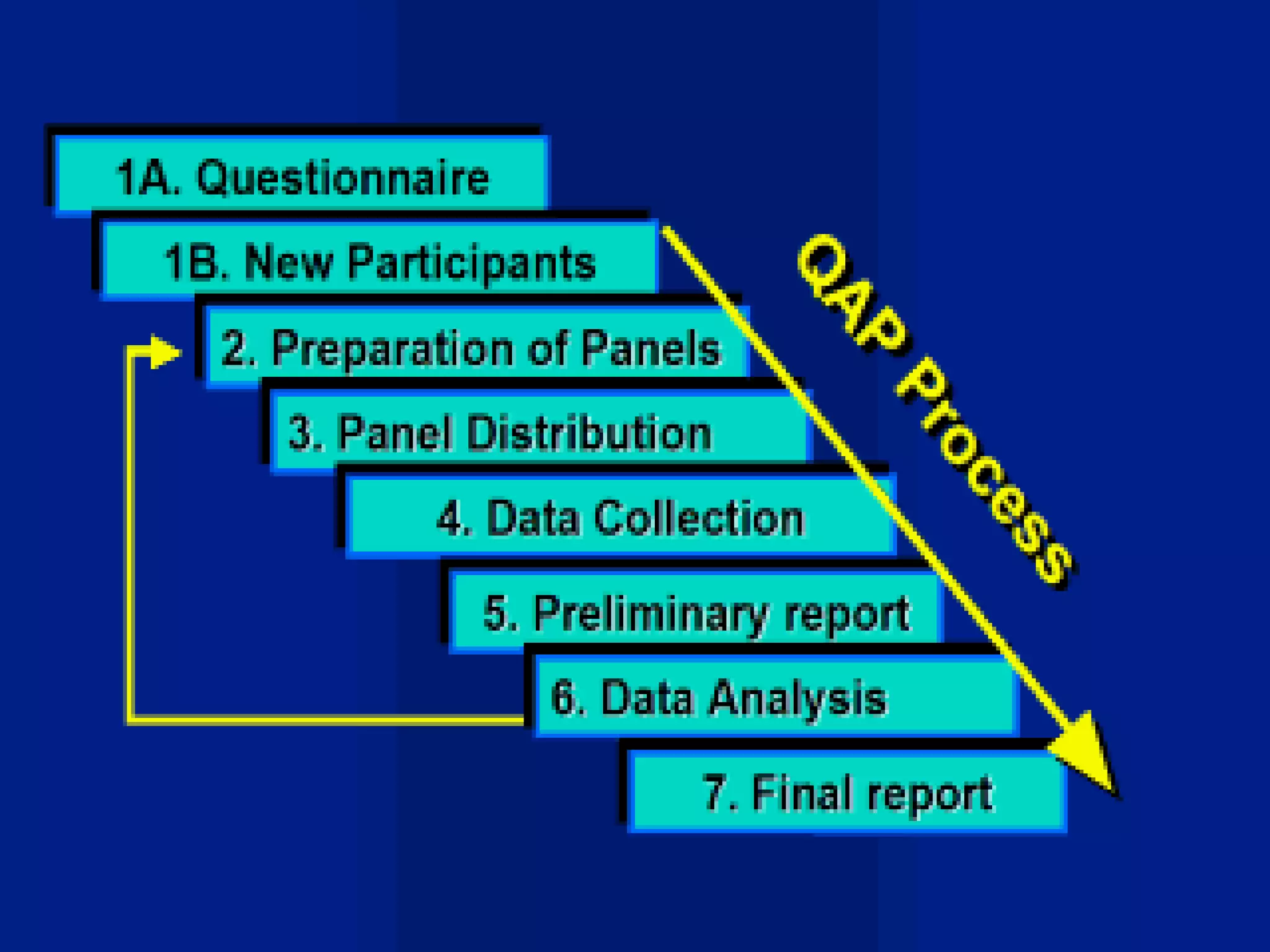
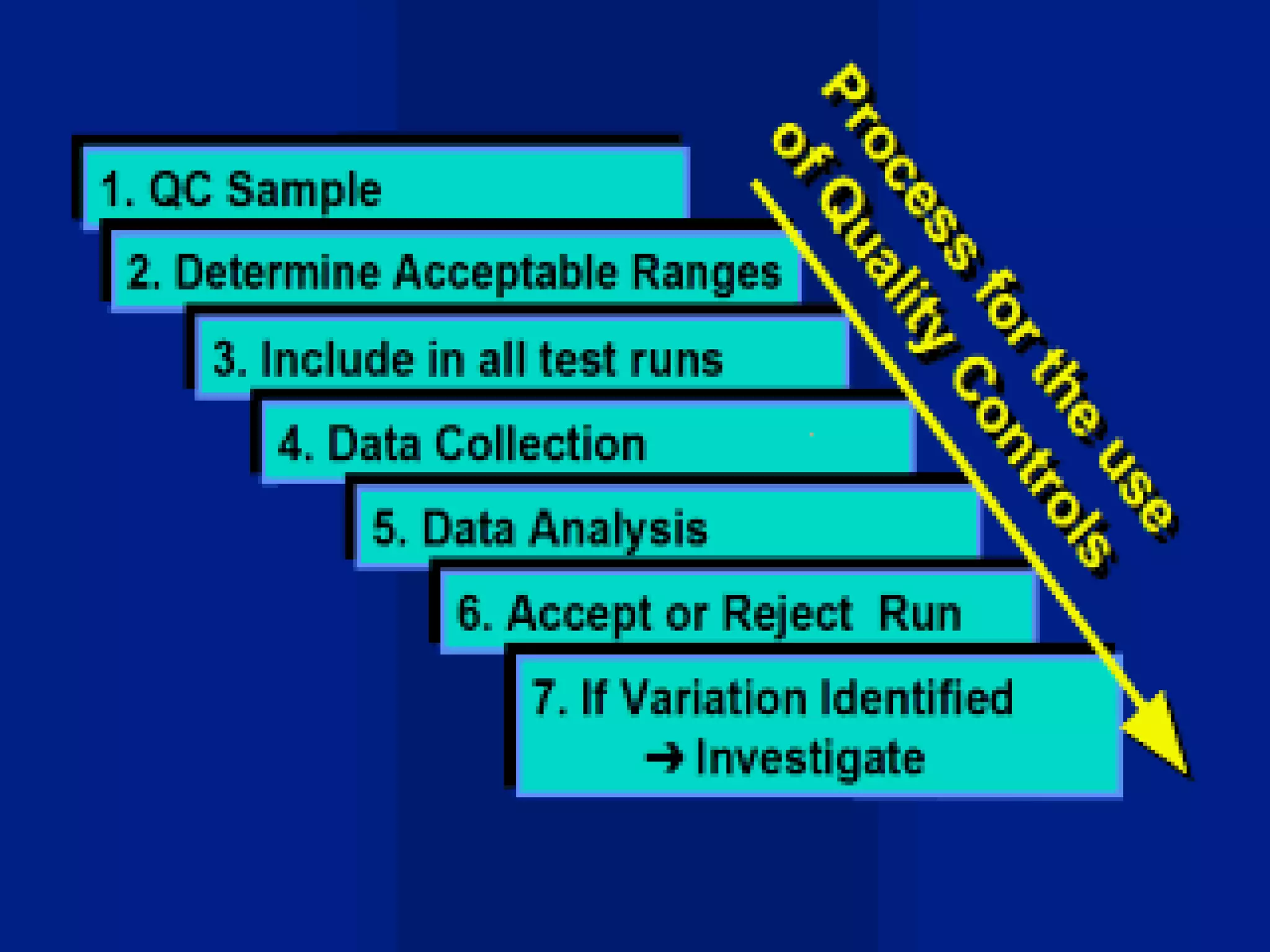
This document discusses quality assurance and provides definitions and explanations of key concepts. It defines quality assurance as activities that provide confidence that quality-related activities are being performed effectively. Quality can refer to technical, philosophical, practical, or metaphysical interpretations. Quality assurance is important in the pharmaceutical industry to ensure high quality standards. Quality objectives and goals help organizations improve and are identified through various inputs and analyses. An effective quality management system uses a process approach.