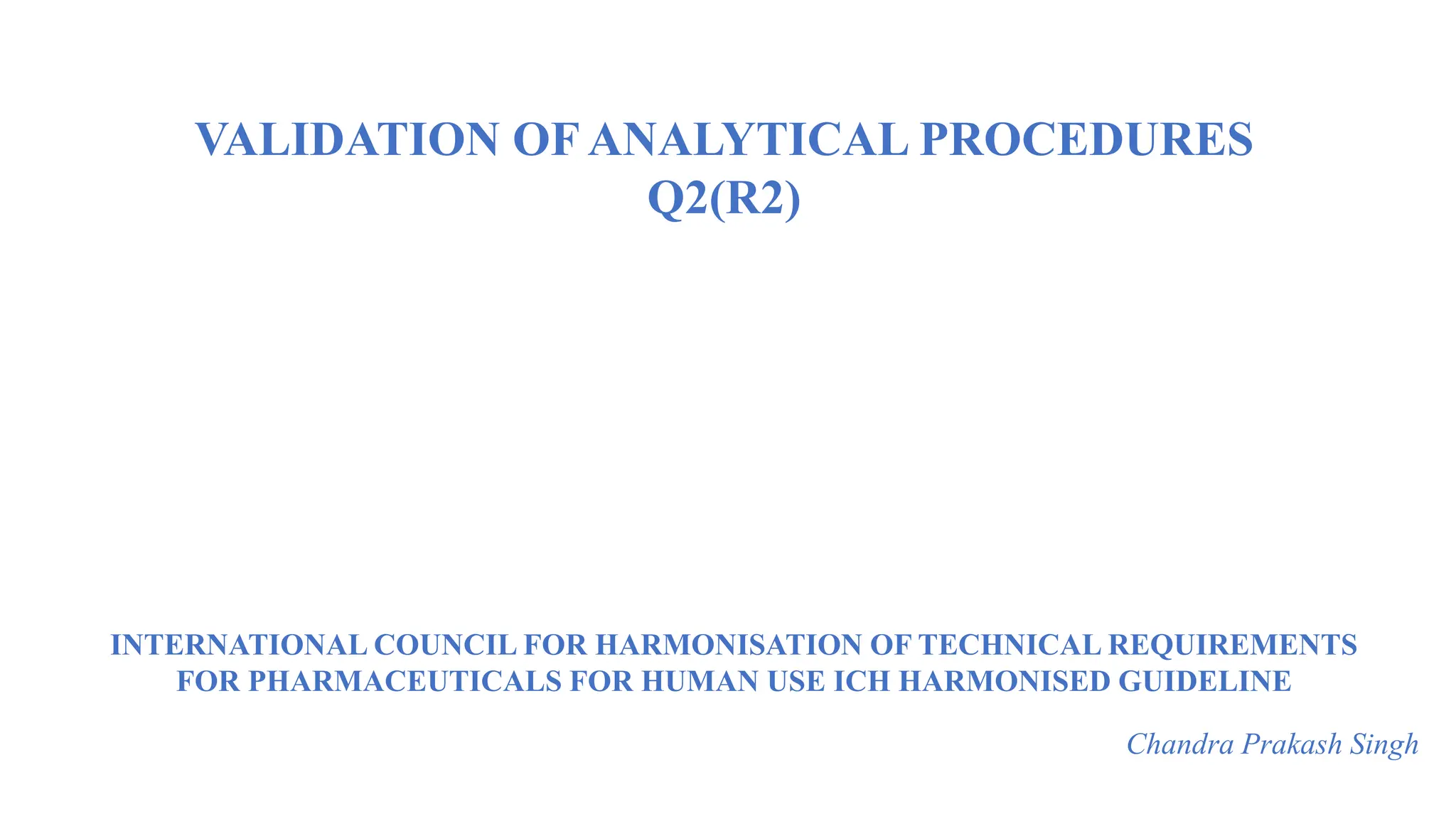
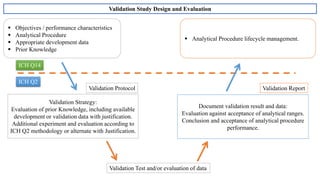
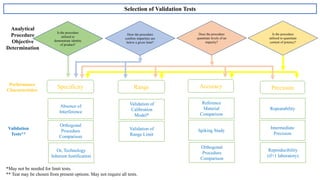
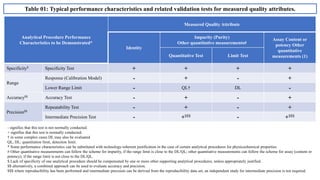
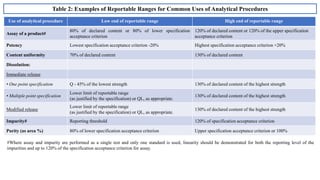
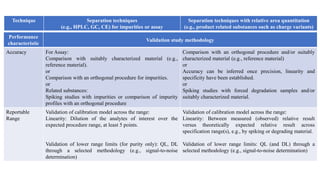
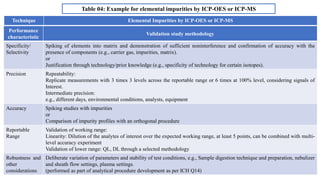
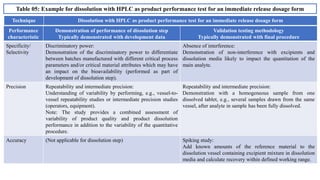
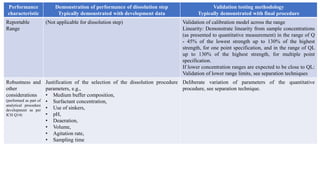
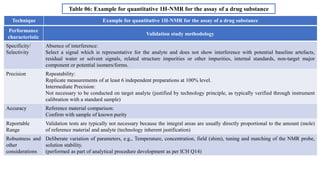
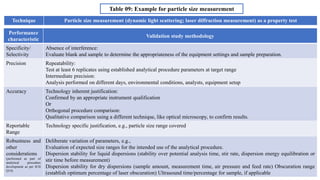
The document provides guidance on validation of analytical procedures according to ICH Q2(R1). It discusses objectives and performance characteristics that should be evaluated. Key aspects of validation include specificity, accuracy, precision, quantitation limit, detection limit, linearity, range, robustness and other validation tests depending on the type of analytical procedure and its intended use for identity, purity, assay, etc. Tables provide examples of validation methodology for common analytical techniques like HPLC, GC, ICP-MS and typical performance characteristics to be demonstrated. The goal of validation is to demonstrate the procedure is suitable for its intended use.