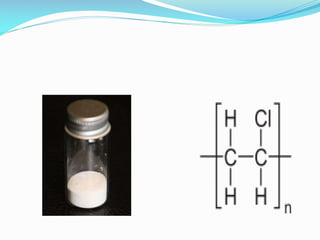
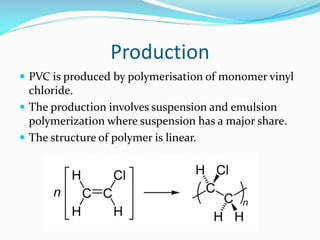
PVC is the third most widely used plastic. It was accidentally discovered in the 19th century when a white solid formed in flasks of vinyl chloride exposed to sunlight. PVC is produced via suspension or emulsion polymerization of vinyl chloride monomer. It has a linear structure and atactic stereochemistry. PVC has applications in pipes, cables, flooring and more due to its low cost, durability and resistance to chemicals and corrosion. However, it has disadvantages like difficulty recycling and sensitivity to heat and UV degradation.