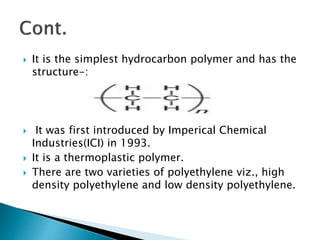
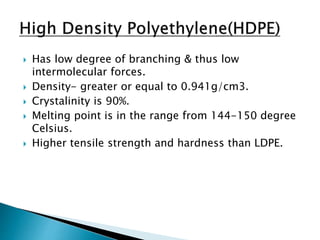
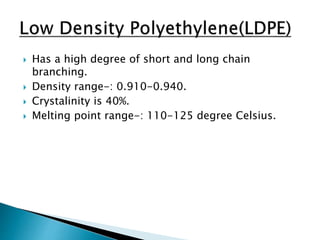
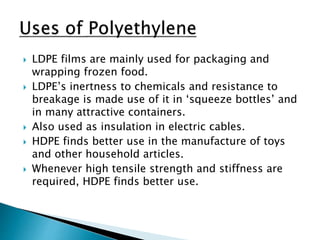
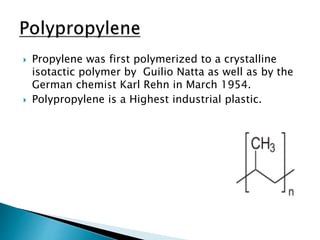
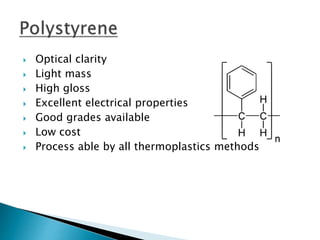
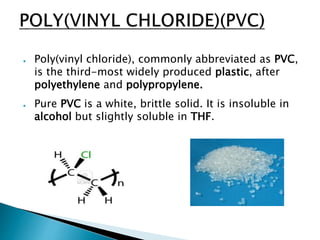
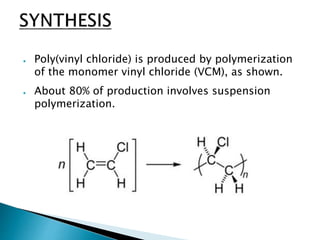
The document discusses polymers and their uses in everyday life. It provides information on different types of polymers like polyethylene, polypropylene, polystyrene, poly(methyl methacrylate), poly(vinyl chloride) and discusses their structures, properties and applications. The document also discusses the synthesis and uses of important polymers.













![ PMMA is a strong and lightweight material. It has a
density of 1.17–1.20 g/cm3,, which is less than
half that of glass.]
It also has good impact strength, higher than both
glass and polystyrene;
however, PMMA's impact strength is still
significantly lower than polycarbonate and some
engineered polymers.
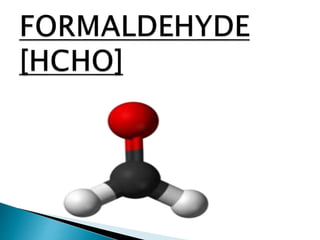
PMMA ignites at 460 °C (860 °F) and burns, forming
carbon dioxide, water, carbon monoxide and low-
molecular-weight compounds, including
formaldehyde.](https://image.slidesharecdn.com/polymersineverydaylife-170704085832/85/Polymers-in-everyday-life-28-320.jpg)








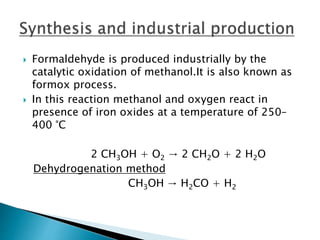
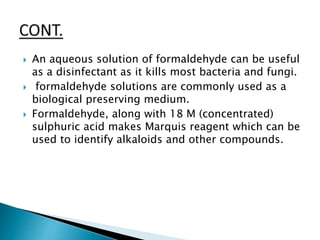
![ Formaldehyde is also a precursor to polyfunctional
alcohols such as pentaerythritol [ C5H12O4 ], which
is used to make paints and explosives.
The textile industry uses formaldehyde-based
resins as finishers to make fabrics crease-resistant.
When treated with phenol, urea, or melamine,
formaldehyde produces, respectively, hard
thermoset phenol formaldehyde resin, urea
formaldehyde resin, and melamine resin. These
polymers are common permanent adhesives used
in plywood and carpeting.](https://image.slidesharecdn.com/polymersineverydaylife-170704085832/85/Polymers-in-everyday-life-46-320.jpg)